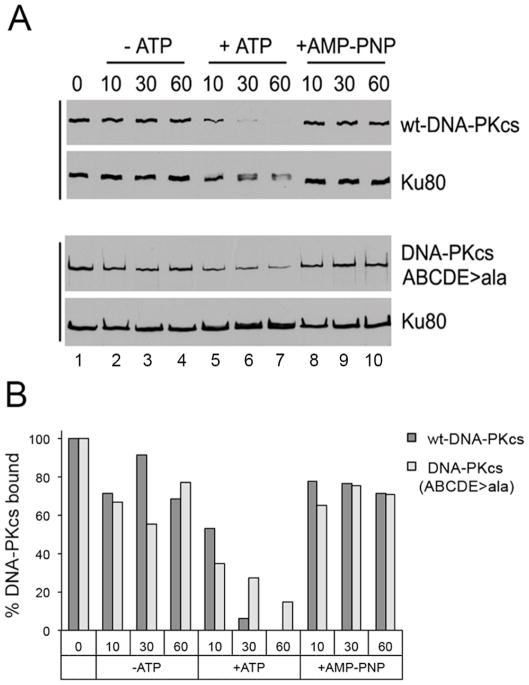
Figure 3. Ablation of ABCDE phosphorylation sites in DNA-PKcs impairs its ability to dissociate from DNA-Ku complexes.
A. Human wild type-DNA-PKcs and DNA-PKcs in which serines and threonines at the ABCDE phosphorylation sites (T2609, S2612, T2620, S2624, T2638, T2647) were mutated to alanine, were stably expressed in DNA-PKcs-deficient V3 rodent cells, purified and incubated with biotin-labelled dsDNA either in the absence of ATP, in the presence of ATP, or the presence of the non-hydrolysable ATP analogue, AMP-PNP as described in (Hammel et al., 2010b). After washing with buffer, beads were resuspended in SDS sample buffer and analysed by SDS PAGE and immunoblot using antibodies to DNA-PKcs or Ku80 as shown. The results show that phosphorylation results in loss of wild-type DNA-PKcs from Ku-DNA complexes whereas more DNA-PKcs is retained with Ku-DNA complexes in the presence of ATP in the autophosphorylation defective mutant (ABCDE sites mutated to alanine). See text and (Hammel et al., 2010b) for details. The upper panel (interaction of wt-DNA-PKcs with Ku and DNA) is taken from Supplementary Figure 10 in (Hammel et al., 2010b), permission applied for. The experiment in the lower panel (purified DNA-PKcs with ABCDE phosphorylation sites mutated to alanine, ABCDE>A) was carried out at the same time as the experiment in the upper panel and equivalent exposures are shown.
B. Bands corresponding to DNA-PKcs in panel A were quantitated using Quantity One software (Biorad), normalized to Ku80 and expressed as a percentage of the sample in lane 1 (incubated with no ATP).