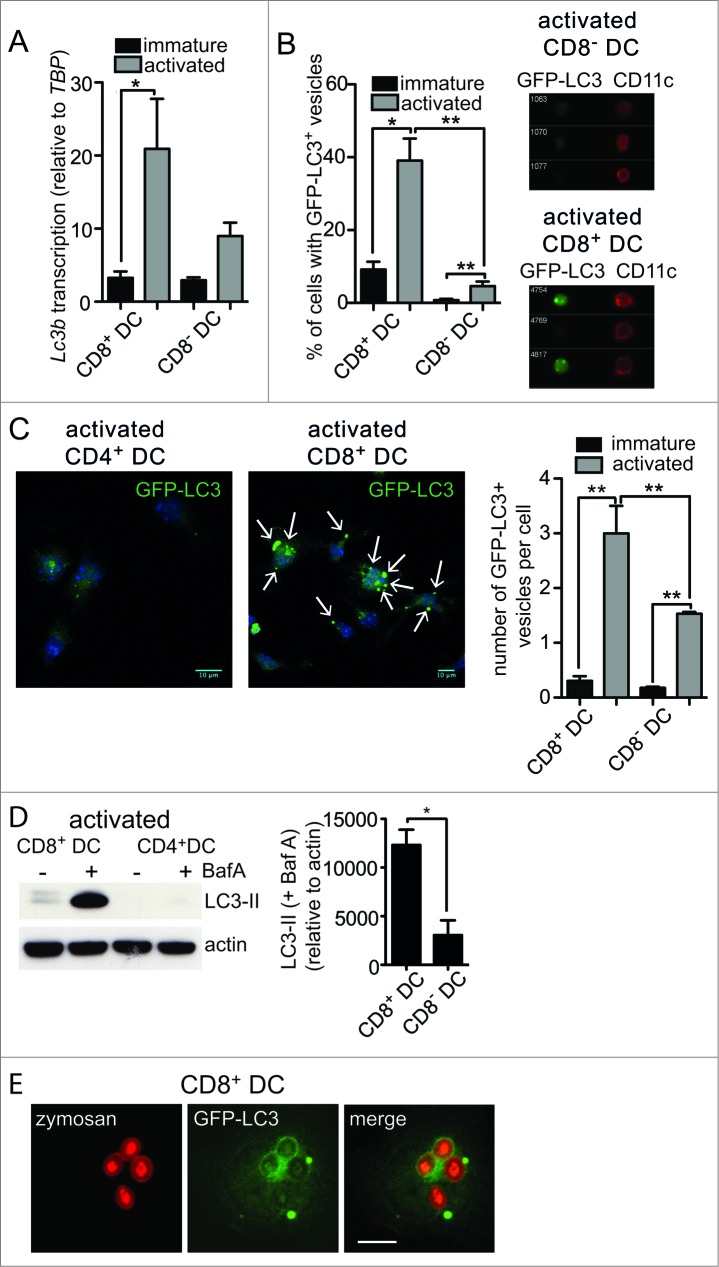
Figure 1.
For figure legend, see page 909.Figure 1 (See previous page). Enhanced autophagy in primary mouse DC with cross-presentation capacity. (A) Splenic CD8+ DC and CD8− DC were purified by flow cytometry. Cells were examined directly ex vivo (immature) or following activation by culture at 37°C overnight (activated). RNA was isolated and quantitative real-time PCR performed to measure Lc3b transcription, relative to the housekeeping gene Tbp (TATA box binding protein). Data are mean ± 1 SEM, pooled from 3 independent experiments, *P ≤ 0.05, one-way ANOVA with Tukey multiple comparison test. (B) Spleen GFP-LC3 DC were cultured at 4°C (immature) or 37°C overnight (activated) and CD8+ DC and CD8− DC analyzed by imaging flow cytometry. Data were pooled from 2 or 3 independent experiments, mean ± 1 SEM, *P ≤ 0.05, one-way ANOVA with Tukey multiple comparison test. (C) Spleen GFP-LC3 CD8+ DC and CD8− DC were cultured overnight (activated). Cells were counterstained with DAPI and imaged by confocal microscopy; scale bar: 10 μm, arrows indicate GFP-LC3 vesicles. Data are from 3 or 4 independent experiments from a total of 126 immature CD8+ DC, 129 immature CD8− DC, 122 activated CD8+ DC, 120 activated CD8 DC, mean ± 1 SEM **P ≤ 0.001, one-way ANOVA with Tukey multiple comparison test. (D) Spleen CD8+ DC and CD8− DC were isolated and cultured overnight (activated). Activated DC were incubated in the presence or absence of 100 nM BafA for 2 h. Cell lysates were subjected to SDS-PAGE and immunoblotted for LC3. Data were pooled from 4 independent experiments, mean ± 1 SEM *P ≤ 0.05, Mann Whitney t test. (E) GFP-LC3 CD8+ DC or CD4+ DC were pulsed with zymosan-Alexa 594 and cultured overnight. Cells were imaged by confocal microscopy; scale bar: 10 μm.