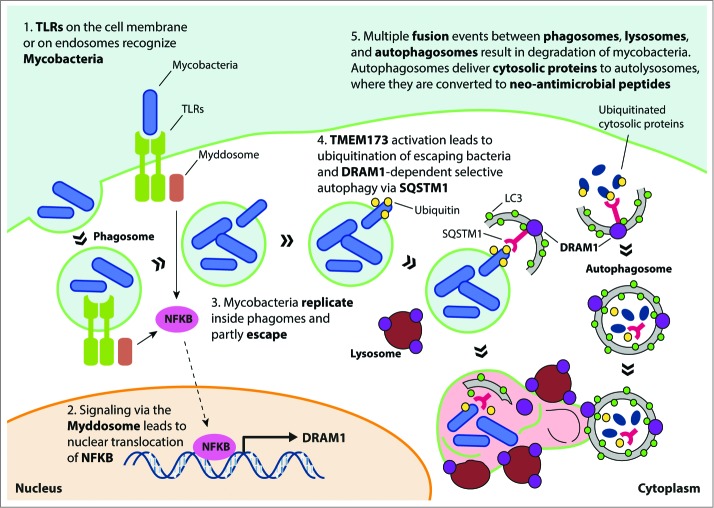
Figure 1.
Model for DRAM1 function in host defense against mycobacteria. TLR recognition of mycobacterial ligands leads to formation of the myddosome (a complex consisting of MYD88-IRAKs-TRAF6), which induces DRAM1 transcription via nuclear translocation of NFKB. Mycobacteria replicate in phagosomes and eventually use RD1-dependent virulence factors to partly escape into the cytoplasm. Release of mycobacterial DNA is thought to trigger activation of TMEM173, ubiquitination of mycobacteria, and recognition by the selective autophagy receptor SQSTM1. DRAM1 protein, localizing on lysosomes and autophagosomes, stimulates formation of autophagosomes and promotes maturation of mycobacteria-containing compartments by facilitating their fusion with lysosomes and autophagosomes that may deliver neo-antimicrobial peptides derived from ubiquitinated cytosolic proteins. DRAM1, DNA-damage regulated autophagy modulator 1; TLR, toll-like receptor; MYD88, myeloid differentiation primary response 88, IRAKs, interleukin-1 receptor-associated kinases; TRAF6, TNF receptor-associated factor 6, E3 ubiquitin protein ligase; NFKB, nuclear factor of kappa light polypeptide gene enhancer in B-cells; SQSTM1/p62, sequestosome 1; TMEM173/STING, transmembrane protein 173.