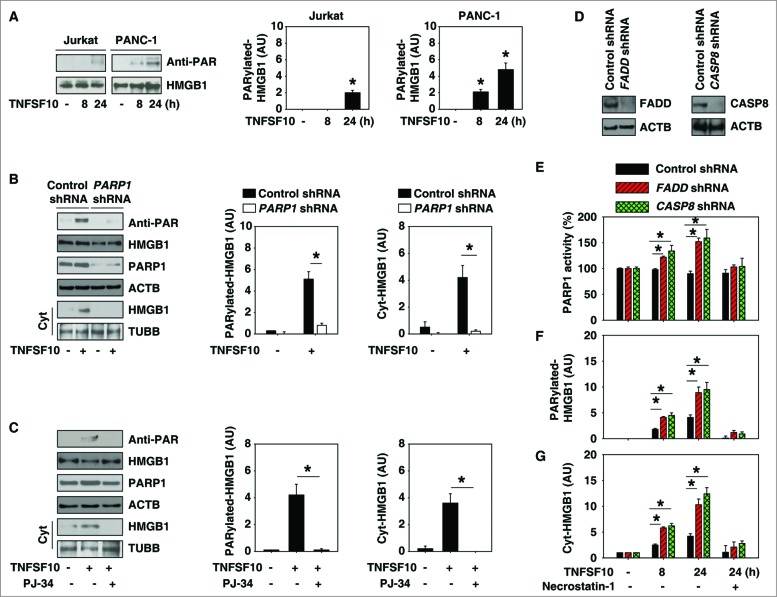
Figure 2.
PARP1 is required for TNFSF10-induced poly-ADP-ribosylation and subsequent cytoplasmic translocation of HMGB1. (A) PANC-1 and Jurkat cells were treated with TNFSF10 (100 ng/ml) for 8 to 24 h. Samples were pulled down with anti-HMGB1 and immunoblotted with anti-PARylation and anti-HMGB1 antibodies. Relative band intensities of HMGB1 PARylation were quantified (*, P < 0.05 versus untreated group). (B) PANC-1 cells were transfected with control shRNA and PARP1 shRNA for 48 h and then treated with TNFSF10 (100 ng/ml) for 24 h. The levels of HMGB1 PARylation and cytoplasmic HMGB1 were assayed (*, P < 0.05). AU, arbitrary unit. (C) PANC-1 cells were treated with TNFSF10 (100 ng/ml) in the absence or presence of PJ-34 (20 μM) for 24 h. The levels of HMGB1 PARylation and cytoplasmic HMGB1 were assayed (*, P < 0.05). AU=arbitrary unit. (D–G) Knockdown of FADD and CASP8 by shRNA (D) increased TNFSF10 (100 ng/ml)-induced PARP1 activity (E), HMGB1 PARylation (F), and HMGB1 cytosolic translocation (G) in PANC-1 cells. In contrast, necrostatin-1 (10 μM), a specific inhibitor of RIPK1, inhibits this process. AU, arbitrary unit.