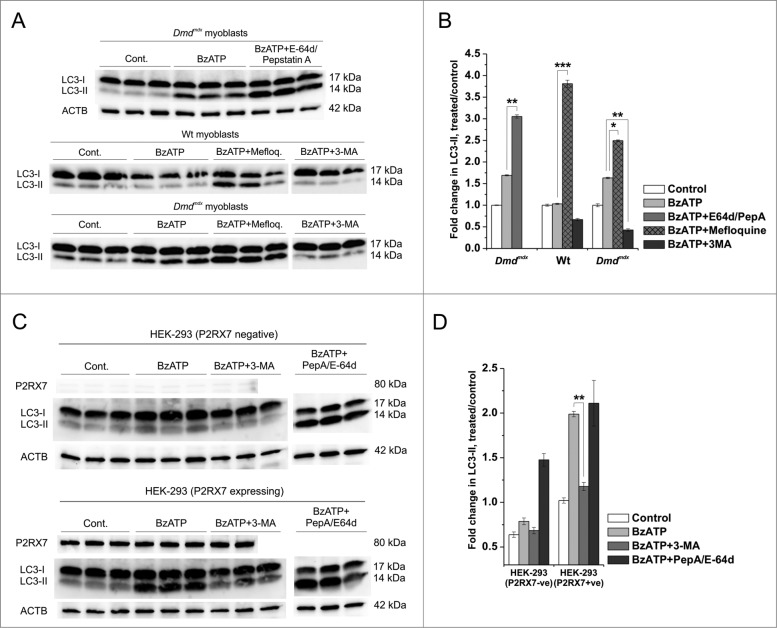
Figure 4.
P2RX7 activation induces autophagic flux in dystrophic myoblasts. (A) Representative protein gel blots of triplicate samples showing LC3-II responses in dystrophic (Dmdmdx) and wild-type (Wt) myoblasts. LC3-II formation invoked following 30 min treatment with 1 mM BzATP was blocked by the autophagy inhibitor 3-methyladenine (3-MA), while lysosomal protease inhibitors E-64d/pepstatin A and mefloquine (Mefloq.), which prevent the degradation of autophagosomes, further enhanced LC3-II accumulation following BzATP treatment. (B) Fold changes of LC3-II conversion as a marker of autophagosome formation. A significant compound increase in LC3-II was observed in cells treated with 1 mM BzATP + E-64d/pepstatin A or mefloquine indicating that specific P2RX7 activation induces the formation of autophagosomes rather than blocks their degradation. Significant decreases in LC3-II observed in cells pretreated with 3-MA may indicate that P2RX7-dependent autophagosome formation occurs via a PtdIns3K-dependent mechanism. (C) P2RX7 expression confers eATP-dependent autophagic flux response to HEK-293 cells. Note no LC3-II response in untransfected (HEK-293-P2RX7 negative, upper panel) and very significant activation in P2RX7-transfected cells (HEK-293-P2RX7 expressing, lower panel) following 30 min treatment with 1 mM BzATP. This effect was blocked by autophagy inhibitor 3-MA (5 mM) and increased by lysosomal protease inhibitors PepA and E-64d (both at 10 μg/ml). (D) Fold changes of LC3-II responses observed in BzATP-treated HEK cells from (C). Mean +/- SE, n = 3, P < 0 .05*, 0.001** and 0.0001***.