Abstract
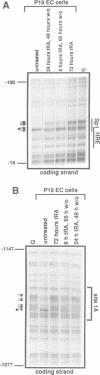
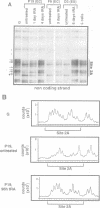
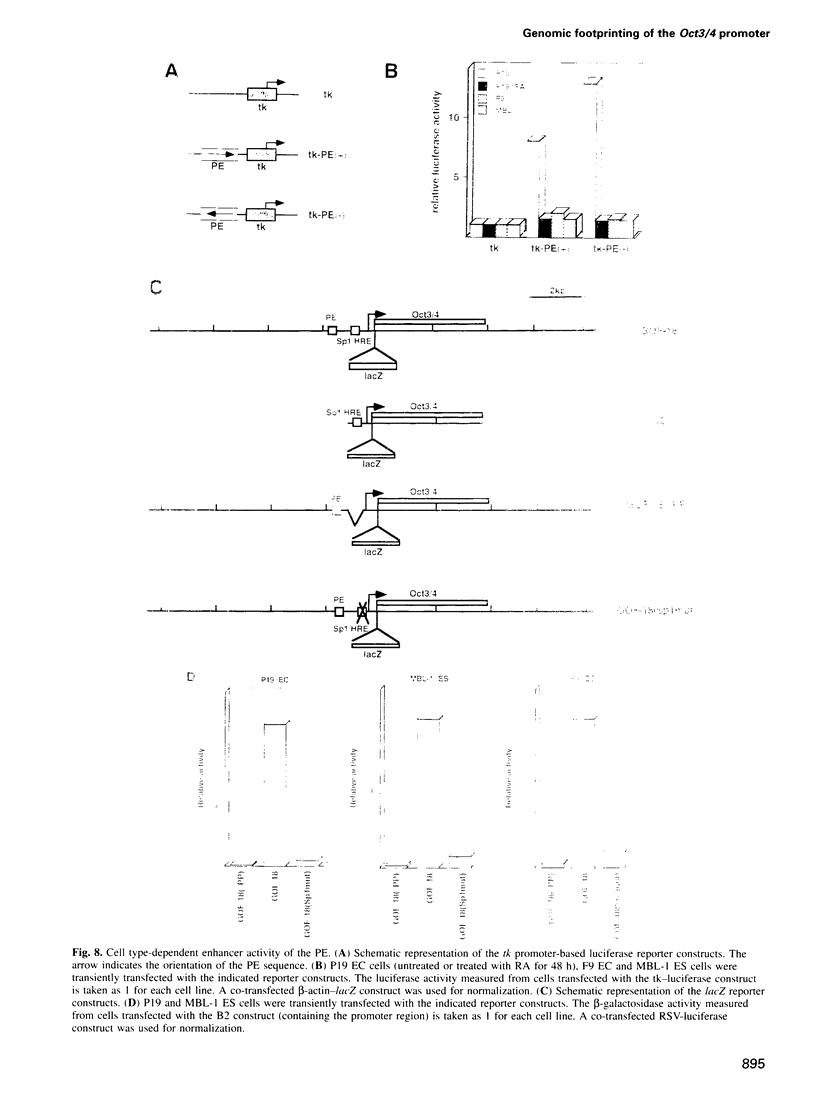
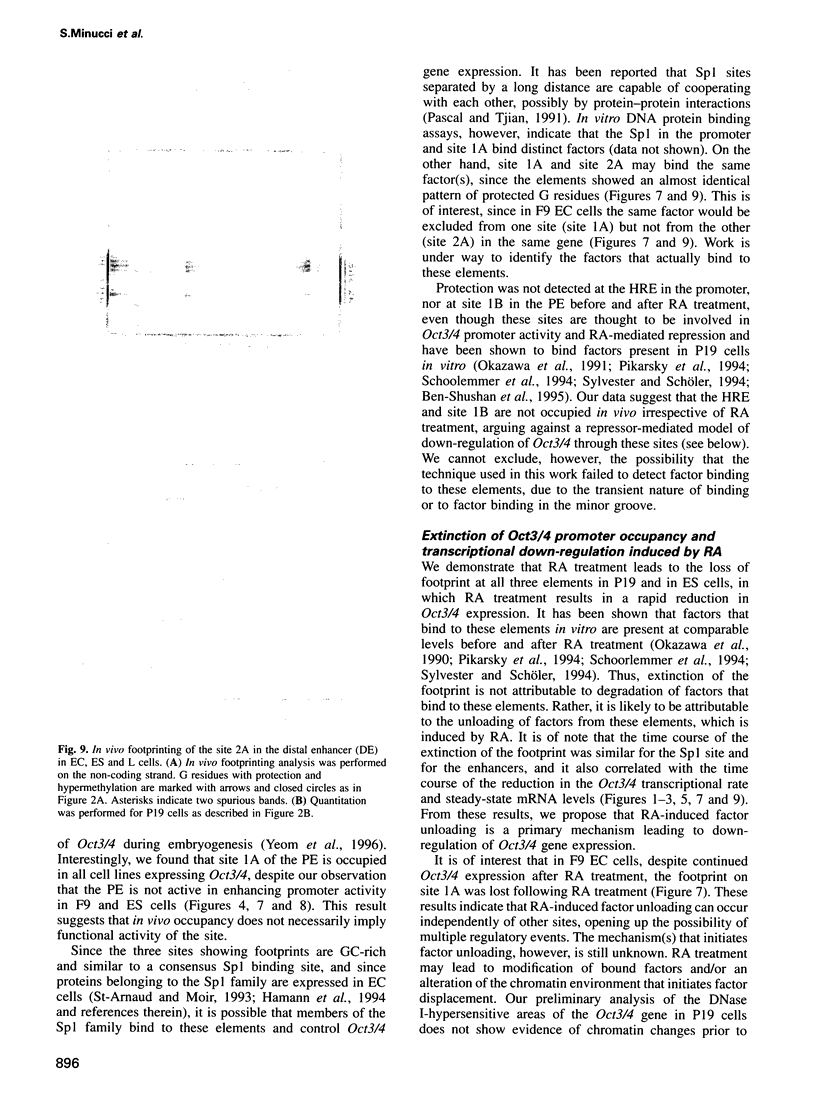
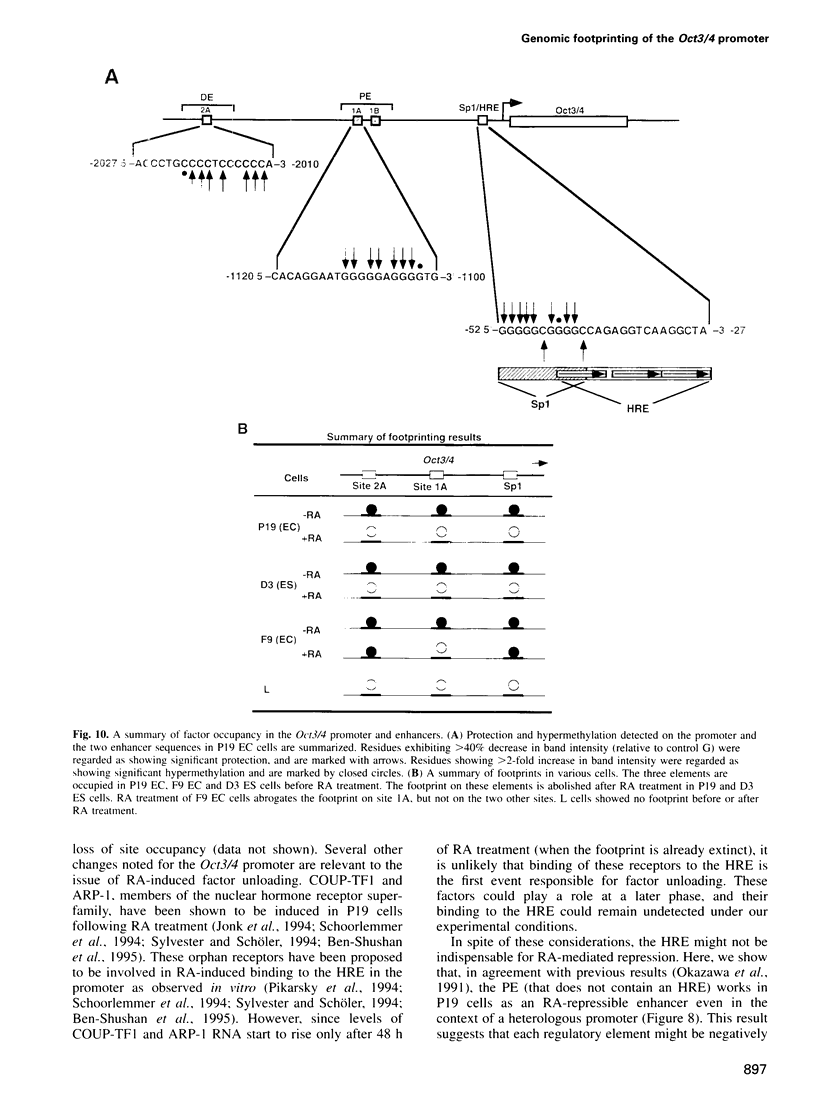
Oct3/4, a hallmark of the earliest stages of embryogenesis, is expressed in undifferentiated embryonal carcinoma (EC) and embryonic stem (ES) cells. Oct3/4 gene expression is dependent on the promoter region, the proximal enhancer and the newly identified distal enhancer. We have analysed in vivo occupancy of these elements. In undifferentiated EC and ES cells, strong footprints were detected at specific sites of all three regulatory elements. These were promptly lost upon RA treatment in ES cells and in P19 EC cells, in parallel with sharply reduced Oct3/4 mRNA levels. Thus, the occupancy of regulatory elements is coupled with Oct3/4 expression, and RA treatment causes coordinated factor displacement, leading to extinction of gene activity. In F9 EC cells, footprint was first abolished at the proximal enhancer. However, this loss of binding site occupancy did not result in a decrease in Oct3/4 mRNA levels. The partial factor displacement seen in F9 EC cells, combined with the observation that EC and ES cells utilize the proximal and distal enhancers in differential manner, indicate the complex pattern of Oct3/4 gene regulation, which could reflect a cell type- and lineage-specific expression of the gene in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Shushan E., Pikarsky E., Klar A., Bergman Y. Extinction of Oct-3/4 gene expression in embryonal carcinoma x fibroblast somatic cell hybrids is accompanied by changes in the methylation status, chromatin structure, and transcriptional activity of the Oct-3/4 upstream region. Mol Cell Biol. 1993 Feb;13(2):891–901. doi: 10.1128/mcb.13.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E., Sharir H., Pikarsky E., Bergman Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Mol Cell Biol. 1995 Feb;15(2):1034–1048. doi: 10.1128/mcb.15.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994 Apr;5(2):115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Danesch U., Hashimoto S., Renkawitz R., Schütz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983 Apr 25;258(8):4750–4753. [PubMed] [Google Scholar]
- Dey A., Minucci S., Ozato K. Ligand-dependent occupancy of the retinoic acid receptor beta 2 promoter in vivo. Mol Cell Biol. 1994 Dec;14(12):8191–8201. doi: 10.1128/mcb.14.12.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Thornton A. M., Lonergan M., Weissman S. M., Chamberlain J. W., Ozato K. Occupancy of upstream regulatory sites in vivo coincides with major histocompatibility complex class I gene expression in mouse tissues. Mol Cell Biol. 1992 Aug;12(8):3590–3599. doi: 10.1128/mcb.12.8.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann L., Bayer K. U., Jensen K., Harbers K. Interaction of several related GC-box- and GT-box-binding proteins with the intronic enhancer is required for differential expression of the gb110 gene in embryonal carcinoma cells. Mol Cell Biol. 1994 Sep;14(9):5786–5793. doi: 10.1128/mcb.14.9.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler B. A., LaRosa G. J., Grippo J. F., Gudas L. J. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989 Dec;9(12):5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonk L. J., de Jonge M. E., Pals C. E., Wissink S., Vervaart J. M., Schoorlemmer J., Kruijer W. Cloning and expression during development of three murine members of the COUP family of nuclear orphan receptors. Mech Dev. 1994 Jul;47(1):81–97. doi: 10.1016/0925-4773(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Linney E. Retinoic acid receptors: transcription factors modulating gene regulation, development, and differentiation. Curr Top Dev Biol. 1992;27:309–350. doi: 10.1016/s0070-2153(08)60538-4. [DOI] [PubMed] [Google Scholar]
- Macleod D., Charlton J., Mullins J., Bird A. P. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 1994 Oct 1;8(19):2282–2292. doi: 10.1101/gad.8.19.2282. [DOI] [PubMed] [Google Scholar]
- Okazawa H., Okamoto K., Ishino F., Ishino-Kaneko T., Takeda S., Toyoda Y., Muramatsu M., Hamada H. The oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991 Oct;10(10):2997–3005. doi: 10.1002/j.1460-2075.1991.tb07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S. L., Peter W., Hess H., Schöler H. R. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994 Nov;166(1):259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Pikarsky E., Sharir H., Ben-Shushan E., Bergman Y. Retinoic acid represses Oct-3/4 gene expression through several retinoic acid-responsive elements located in the promoter-enhancer region. Mol Cell Biol. 1994 Feb;14(2):1026–1038. doi: 10.1128/mcb.14.2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer J., van Puijenbroek A., van Den Eijnden M., Jonk L., Pals C., Kruijer W. Characterization of a negative retinoic acid response element in the murine Oct4 promoter. Mol Cell Biol. 1994 Feb;14(2):1122–1136. doi: 10.1128/mcb.14.2.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Balling R., Hatzopoulos A. K., Suzuki N., Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989 Sep;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Dressler G. R., Balling R., Rohdewohld H., Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990 Jul;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ruppert S., Suzuki N., Chowdhury K., Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990 Mar 29;344(6265):435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Moir J. M. Wnt-1-inducing factor-1: a novel G/C box-binding transcription factor regulating the expression of Wnt-1 during neuroectodermal differentiation. Mol Cell Biol. 1993 Mar;13(3):1590–1598. doi: 10.1128/mcb.13.3.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester I., Schöler H. R. Regulation of the Oct-4 gene by nuclear receptors. Nucleic Acids Res. 1994 Mar 25;22(6):901–911. doi: 10.1093/nar/22.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., Wang L., Wu T. C. Different response to retinoic acid of two teratocarcinoma cell lines. Exp Cell Res. 1995 Aug;219(2):392–398. doi: 10.1006/excr.1995.1244. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]