Figure 8.
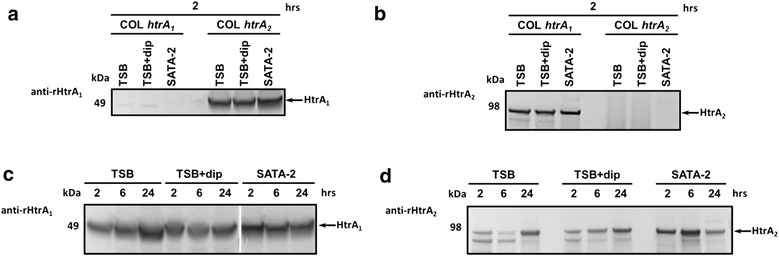
Expression of WT HtrA proteins in S. aureus. Immunoblot analysis using polyclonal anti-rHtrA1 (a, b) or anti-rHtrA2 (c, d) sera was performed on cell lysates of different S. aureus strains: each htrA mutants of strain COL (designated as COL htrA 1 and COL htrA 2 [57], a, b) and WT strain Lowenstein (c, d). All strains were grown in two media (TSB, SATA-2) and in the first line, under two different conditions (TSB, TSB + dipyridyl corresponding to the addition of chelator), till different growth phases: the exponential phase (2 h) for htrA mutants of strain COL (a, b) and, for WT strain Lowenstein (c, d), the exponential (2 h), early stationary (6 h) and late stationary (24 h) phases. HtrA1 was found to be completely stable (a, c), whereas HtrA2 protein underwent a limited proteolysis giving rise to one minor degradation product of high molecular weight in the cells (b, d), as confirmed by the absence of proteolytic products in culture supernatants. S. aureus HtrA1 and HtrA2 thus behave differently from Bacillus subtilis HtrA (YkdA) protein (YkdA) which is known to be released into the supernatant as a processed form, devoid of its transmembrane domain [73].
