Abstract
Background context
Length of hospital stay (LOS) after lumbar spine surgery (LSS) can be affected by many factors. However, few studies have evaluated predictors of LOS, and all have used limited number of variables as predictors.
Purpose
To identify pre-surgical, surgical, and post-surgical predictors of LOS following LSS.
Study Design/Setting
Retrospective review of consecutive patients who had LSS at the (Blinded) Hospital from October, 2008 to April, 2012.
Patient Sample
593 patients who underwent LSS consisting of laminotomy, laminectomy, or arthrodesis.
Outcome Measures
Dependent variable: LOS. Multiple pre-surgical, surgical, and post-surgical variables were extracted from patients’ medical records and considered as possible predictors (independent variables) of LOS.
Methods
Potential predictors that were significantly correlated with LOS were used as indicators to construct three latent factors; pre-surgical, surgical, and post-surgical, which were in turn used to predict LOS in a structural equation model (SEM).
Results
The average LOS was 4.01±2.73 days. The pre-surgical factor was indicated by age (61.97±14.49 years), prior level of function (60.5% were totally independent), prior hemoglobin level (13.70±1.36 mg/dl), and use of assistive devices (60% were assistive device users). The surgical factor was indicated by severity of illness (50.2% had minor disease severity), presence of complications (1.9%), and stay in an intensive care unit (4.0%). The post-surgical factor was indicated by post-surgical walking distance (166.43±175.75 feet), level of assistance during walking (5.18±0.81 out of 7 points), balance scores (6.18 ±1.82 out of 10 points), and bed mobility and transfer dependency scores (9.81± 1.99 out of 14 points). These three latent factors explained 47% of variation in LOS.
Conclusion
Post-surgical factors predicted the highest variation in LOS in comparison to pre-surgical and surgical factors and should be taken into consideration for discharge planning. Post-surgical factors are related to the patient's function, modifiable with rehabilitation and can be improved to shorten LOS. Inclusion of more reliable and standardized pre-surgical variables could improve the predictability of the model.
Keywords: Lumbar surgery, length of stay, functional assessment, structural equation model
Introduction
Lumbar spine surgery (LSS) such as laminectomy or fusion is commonly performed for treatment of stenosis, spondylosis, spondylolisthesis, disc herniation, or other causes of low back pain that requires hospital stay. The rate of LSS in the United States is the highest in the world, with high surgery costs and related post-surgical care [1, 2]. Hospital costs are estimated to be 31% of total health costs in the United States [3].Thus, reducing length of hospital stay (LOS) following LSS may be cost saving, if decreased LOS also results in lowering fees charged by hospitals.
LOS is a complex construct that depends on many surgical and non-surgical factors. Only three studies have investigated predictors of LOS following LSS using retrospective analysis methods. One study used limited variables [3], whereas others included patients with revision LSS only [4] or investigated surgical factors as predictors of intensive care unit (ICU) LOS [5]. These studies collectively identified many variables that correlated with LOS but did not determine their unique contribution in predicting LOS. Some of the significant predictors of LOS were age, pre-operative hemoglobin, pre-operative narcotic use, American Society of Anesthesiologists score, type of surgery, fluid balance, mean percentage of fraction of inspired oxygen, volume of fluid transfused, post-operative creatinine level, post-operative pain intensity, dependency score, number of physical therapy (PT encounters), and post-operative complications [4-9]. Variables such as marital status, home situation, pre-operative use of walking aids, clinical diagnosis, and post-surgery physical mobility have been investigated to predict LOS following other orthopedic surgeries [10-14], but have not been studied as possible predictors of LOS following LSS.
Increasing sample size and inclusion of more variables may improve the prediction accuracy and explain a higher percentage of variability in LOS. One of the major challenges to studying predictors of LOS is that many variables are multi-dimensional and correlated with each other, resulting in inconsistent results and difficulty in selecting main predictors. A structural equation model (SEM) allows for testing relationship among many variables and construction of latent factors such as pre-surgical, surgical and post-surgical, as latent factors cannot be directly constructed by indicators (manifest variables) [15]. The SEM may also be a useful method of analysis to illustrate the factors that predict LOS while showing correlations between these factors [16].
The purpose of this retrospective study was to investigate clinical predictors of LOS following LSS. The second aim of this study was to determine which group of significant predictors (pre-surgical, surgical, or post-surgical) explained the highest variation in LOS following LSS. Several potential predictors that were significantly correlated with LOS were used as indicators to construct three latent factors of interest; pre-surgical, surgical, and post-surgical. These factors were used to predict LOS, using a SEM. We hypothesized that the factors constructed from the significant indicators would have a significant direct effect on LOS.
Methods
Data source
We extracted de-identified data from the (blinded) Hospital's electronic medical records (Epic Corporation) and other administrative, research, and public sources. The study received exempt determination from the Institutional Review Board for the use of de-identified data.
Study Cohort
We reviewed patient records of those who underwent LSS at the (blinded) Hospital between October, 2008 and April, 2012. We identified our cohort of interest on the Healthcare Enterprise Repository for Ontological Narration (HERON) system using the i2b2 query and analysis tool [17] with specific CPT codes (see appendix A) to select patients who had posterior LSS consisting of laminotomy, laminectomy, or fusion (arthrodesis). Medical records for patients 18 years or older who had formal LOS data from the billing records and inpatient physical therapy (PT) assessment data were included. Medical records were excluded for patients with history of neoplasm, intraspinal abscess, spinal deformity (i.e. scoliosis, kyphoscoliosis), spine fractures, vertebroplasty, osteomyelitis, and cauda equina syndrome. Based on these criteria, 601 records were identified.
Selection of covariates
Using the i2b2 query and analysis tool, covariates of interest were selected based on previous similar studies on LOS [4-6], predictors of other outcomes following LSS [18-27], and predictors of LOS following total knee or hip replacement [28-30]. Socio-demographic data were extracted directly from the Epic system. We used the date of the surgery as a reference date between pre-surgery and post-surgery. Information about diagnosis and impression of the cause of low back pain was obtained from physicians’ notes.
We selected the closest pre-surgical complete blood count (CBC) lab tests (at most 2 weeks before the surgery) to indicate pre-surgical hemoglobin and hematocrit levels. We selected the CBC test measured one day after the surgery to indicate post-surgical hemoglobin and hematocrit levels. The total volume of fluid resuscitation was calculated as sum of the volumes of crystalloids and colloid.
PT inpatient flowsheets were accessed to extract PT assessment and treatment data, and documentation to identify patients’ home type, living situation, and prior level of function (PLOF). PLOF was determined based on the level of assistance needed in mobility and activities of daily living as reported by the patient (fully independent, independent in community with limitation, and independent at household level with or without assistance). Post-operative functional dependency score was evaluated by the 8-point functional independence measure (FIM) scale (1= total assistance, 7= total independence) [31] and obtained from PT flowsheets. FIM scores for bed mobility and transfer were combined as the final dependency score. Gait distance in feet and the level of assistance needed during gait training were collected. Balance was assessed on an 11-point (the higher the score, the better the balance) balance scale [32]. Sitting and standing balance scores were averaged to create one combined score (balance score). Average of gait distance, dependency score, and balance score during the LOS was calculated.
Data related to LOS, comorbidities, complications, severity of illness, admission day of the week, and ICU LOS were obtained from the hospital billing records within HERON. The All Patient Refined-Diagnosis Related Group (APR-DRG) severity of illness was calculated based on primary and secondary discharge diagnoses, age, and pre-existing medical conditions [33]. Severity of illness was rated as minor, moderate, major, and severe. Comorbidities were any of these conditions: cerebrovascular disease, chronic pulmonary disease, cardiovascular disorders, connective tissue disease, dementia, hemiplegia, leukemia, malignant lymphoma, myocardial infarction, peripheral vascular disease, ulcer disease, endocrine disorders, liver disease, renal disease, malignant tumor, depression, anemia, obesity, fluid and electrolytes disorders, psychosis, alcohol and drug abuse [34]. Finally, complications were any of these conditions: acute myocardial infarction, nosocomial pneumonia, sepsis, wound infection, implant or graft complication, aspiration pneumonia, or gastrointestinal hemorrhage. We used the number of comorbidities and complications as covariates.
Data management and statistical analysis
The univariate normality distribution of continuous variables was tested, and transformation was performed whenever needed (square root transformation for gait distance (sqrt (gait) and natural log transformation of LOS (Ln (LOS)). Univariate outliers were screened using Q-Q plots, and multivariate outliers were investigated using Mahalanobis d-squared distance [35]. The multivariate normality distribution of the model was tested using Mardia's coefficient [36].
We used SPSS 20.0 (SPSS Inc. Chicago, IL) for descriptive statistics and correlational analyses, and SPSS Amos 20.0 (SPSS Inc. Chicago, IL) for SEM analysis. The SEM consisted of Ln (LOS) as the dependent variable to be regressed on three latent factors of interest indicated by multiple manifest (indicator) variables as follows:
Pre-surgical factor: Age, sex, race, ethnicity, body mass index (BMI), marital status, living situation, type of home, use of assistive devices, PLOF, hemoglobin and hematocrit levels before surgery, and number of comorbidities.
Surgical factor: Type of surgery, previous LSS, admission day of the week, diagnosis, severity of illness, ICU LOS, complications, and total volume of fluid resuscitation.
Post-surgical factor: Gait distance, gait assistance, combined dependency scores of bed mobility and transfer, balance score, hemoglobin and hematocrit levels after surgery, and post-operative pain intensity and pain location.
We estimated loadings (regression weight) and modification indices to examine the best fitting indicators of the factors prior to SEM testing and excluded manifest variables according to each variable's contribution to overall model fitting [15]. Variables that generated loading less than 0.3 standardized estimates (λ of composite scores of the manifest variables) were dropped from the factors. The overall reliability of the factor was indicated by an alpha coefficient >0.7. We set the threshold of modification indices at 4.0. Significance (p<0.05) was determined by non-parametric bootstrapping of 2000 samples.
Results
Of the 601 medical records, eight cases were deleted as multivariate outliers, resulting in 593 cases. Table 1 shows the summary of the covariates of the 593 cases. The original data from the medical records were analyzed without imputation.
Table 1.
Descriptive statistics for all the covariates extracted from 593 medical records
| Variable | Mean (SD), % | N |
|---|---|---|
| Length of stay (days) | 4.01 (2.73) | 593 |
| Age (yrs) | 61.97 (14.49) | 593 |
| Gender | 593 | |
| • Male | 52.3% | |
| • Female | 47.7% | |
| Race | 593 | |
| • White | 83.2% | |
| • Non-white | 16.8% | |
| Ethnicity | 593 | |
| • Non-Hispanic | 97.2% | |
| • Hispanic | 2.8% | |
| Marital status | 593 | |
| • Married | 66.0% | |
| • Divorced/separated/widowed/single | 34.0% | |
| Body Mass Index (kg/m2) | 31.95 (6.59) | 593 |
| Hemoglobin before surgery (mg/dl) | 13.70 (1.36) | 585 |
| Hematocrit before surgery (%) | 40.35 (3.88) | 586 |
| Type of home | 544 | |
| • House | 91.6% | |
| • Apartment | 6.3% | |
| • Other | 2.1% | |
| Home situation | 530 | |
| • Lives with family | 73.8% | |
| • Has assistance at home | 10.1% | |
| • Lives alone | 16.1% | |
| Prior level of function (PLOF) | 525 | |
| • Independent Mobility at Household Level with or without e/assistance | 12.1% | |
| • Independent Mobility in Community w/ device or Endurance Limitations | 27.4% | |
| • Independent | 60.5% | |
| Assistive devices used | 543 | |
| • None | 40.0% | |
| • 1 or 2 points | 31.0% | |
| • 3 or 4 points | 28.9% | |
| Total number of comorbidities | 593 | |
| • 0 | 45.2% | |
| • 1 | 23.2% | |
| • 2 | 15.3% | |
| • 3≤ | 16.3% | |
| Surgery classification | 593 | |
| • Laminotomy | 18.5% | |
| • Laminectomy alone or with laminotomy | 41.8% | |
| • Fusion with laminotomy or laminectomy or both | 39.7% | |
| Previous surgeries | 593 | |
| • No | 95.5% | |
| • Yes | 4.5% | |
| Diagnosis | 593 | |
| • Lumbago | 11.3% | |
| • Spinal stenosis of lumbar region | 58.4% | |
| • lumbosacral neuritis or radiculitis, sciatica/ lumbar intervertebral disc | 29.1% | |
| • Lumbosacral spondylosis without myelopathy | 1.2% | |
| Total infusion (mL) | 2477.98 (1189.06) | 497 |
| Severity of illness | 593 | |
| • Minor | 52.2% | |
| • Moderate | 41.6% | |
| • Severe | 6.2% | |
| Admission day of the week | 593 | |
| • Sunday, Monday. Tuesday, Wednesday | 36.0% | |
| • Thurs, Friday, Saturday | 57.5% | |
| Complication/s | 593 | |
| • No | 98.1% | |
| • Yes | 1.9% | |
| Intensive care unit length of stay (days) | 593 | |
| • 0 | 96.0% | |
| • 1 | 2.3% | |
| • 2 ≤ | 1.7% | |
| Pain intensity (0-10 pain scale) | 5.19 (1.31) | 593 |
| Pain location | 593 | |
| • Back only | 73.8% | |
| • Thigh and buttock | 11.1% | |
| • Leg and feet | 15.1% | |
| Dependency score | 9.81 (1.99) | 525 |
| Balance | 6.18 (1.82) | 560 |
| Gait assistance | 5.18 (0.81) | 570 |
| Gait distance | 166.43 (175.75) | 570 |
| Hemoglobin after surgery (mg/dl) | 11.36 (1.54) | 579 |
| Hematocrit after surgery (%) | 33.35 (4.41) | 583 |
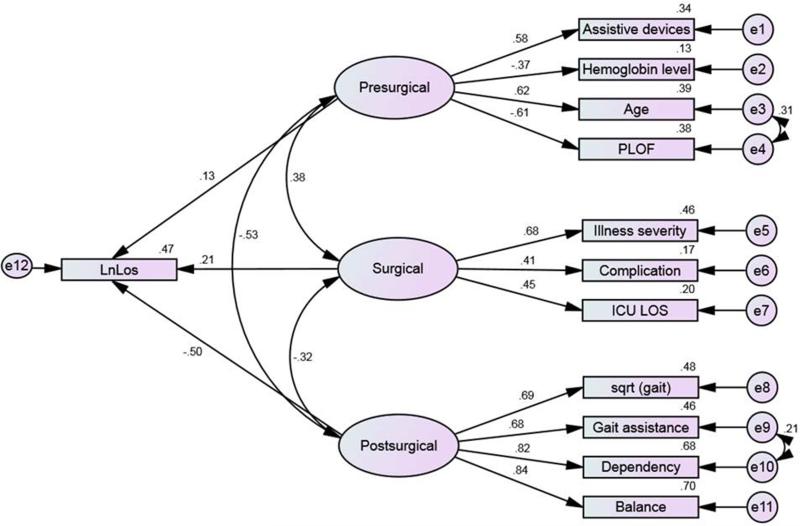
Appendices B, C and D show the correlation matrices between all covariates. LOS was significantly correlated with all variables except pain intensity, admission day of the week, ethnicity, marital status, and history of previous spine surgery. Thus, these variables were not considered as indicators (potential predictors) in constructing the latent factors (pre-surgical, surgical, and post-surgical). The modification indices (>4.0) indicated the need to covariate age with PLOF, gait assistance with dependency score and the three latent factors to correct the fitness of the model (Figure 1). Figure 1 shows the final SEM constructed from the three latent factors (pre-surgical, surgical, and post-surgical). Test of fit Bollen-Stine [37] (p = 0.24) and relative fit tests of NFI=0.97, IFI=0.99, CFI= 0.99, and RMSEA= 0.02 (90% CI=0.00 – 0.04) indicated that our model relatively fits the data [38] (Table 2). Table 2 demonstrates the correlation between all covariates that were used to construct the final model.
Figure 1.
Structural Equation Model for the length of hospital stay (LOS) constructed in Amos. Quantities near paths and squares are standardized loadings and squared multiple correlations, respectively. Degrees of freedom=47, e=error. Ln (LOS) is the dependent variable. Variables presented in ellipse are latent factors.
Table 2.
Correlation matrices showing correlation coefficients between covariates used in the final structural equation model
| Mean (SD) | LOS | Assistive device | Hemoglobin level | Age | PLOF | Severity of illness | Complication | ICU LOS | Gait distance | Dependency | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOS | 4.01(2.73) | 1 | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Assistive devices | 1.27(1.38) | .223± | 1 | -- | -- | -- | -- | -- | -- | -- | -- |
| Hemoglobin level | 13.70(1.36) | −.175± | −.19± | 1 | -- | -- | -- | -- | -- | -- | -- |
| Age | 61.97(14.49) | .24± | .35± | −.26± | 1 | -- | -- | -- | -- | -- | -- |
| PLOF | 3.40(.91) | −.32± | −.39± | .18± | −.19± | 1 | -- | -- | -- | -- | -- |
| Severity of illness | .55(.64) | .32± | .18± | −.12± | .15± | −.21± | 1 | -- | -- | -- | -- |
| Complication | .02(.14) | .23± | −.03 | −.02 | .06 | −.01 | .28± | 1 | -- | -- | -- |
| ICU LOS | .07(.44) | .19± | .05 | −.07 | .11* | −.17± | .30± | .23± | 1 | -- | -- |
| Gait distance | 166.43(175.8) | −.39± | −.19± | .20± | −.14± | .18± | −.12± | −.07 | −.11± | 1 | -- |
| Dependency score | 9.81(1.99) | −.47± | −.23± | .22± | −.27± | .26± | −.21± | −.08 | −.14± | .54± | 1 |
| Balance score | 6.18(1.82) | −.46± | −.27± | .20± | −.29± | .26± | −.20± | −.07 | −.20* | .57± | .69± |
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
LOS: Length of stay, PLOF: Prior level of function, ICU LOS: intensive care unit length of stay
The model posited that pre-surgical, surgical, and post-surgical factors had significant direct effects on the Ln (LOS). The indicator (manifest) variables had significant direct effects on these latent factors (Table 3).
Table 3.
Unstandardized estimates of the direct effects resulting from the structural equation model analysis
| Measurement model | Parameter | Estimates | Bootstrap S.E. | Bias-corrected, 95% CI | p |
|---|---|---|---|---|---|
| Presurgical | Use of assistive devices | 1.00 | |||
| Hemoglobin level | −1.04 | .15 | −1.36 - −.78 | .001 | |
| Age | 18.74 | 2.18 | 15.13 – 23.99 | .001 | |
| PLOF | −1.16 | .14 | −1.44 - −0.91 | .001 | |
| Surgical | Severity of illness | 1.0 | |||
| Complication | .13 | .06 | .05 - .29 | .001 | |
| ICU LOS | .20 | .08 | .1 - .409 | .001 | |
| Postsurgical | Sqrt (gait distance) | 1.00 | |||
| Gait assistance | .12 | .01 | .10 - .14 | .001 | |
| Dependency score | .36 | .03 | .32 - .42 | .001 | |
| Balance combined score | .33 | .02 | .30 - .37 | .001 | |
| Structural model | |||||
| Ln (Los) | Presurgical | .18 | .08 | .01 - .32 | .044 |
| Surgical | .33 | .12 | .149 - .64 | .001 | |
| Postsurgical | −.08 | .01 | −.09 - −.060 | .001 |
PLOF: prior level of function, LOS: length of stay, ICU LOS: intensive care unit length of stay, S.E: standard error
Unstandardized effects
Pre-surgical
The direct effect of the pre-surgical factor on Ln (LOS) was 0.18 (p=0.04, 95% CI= 0.01 – 0.32), which is equal to 1.19 (95% CI=1.01– 1.38) on LOS, suggesting that the LOS is increased by 1.19 times when the pre-surgical factor is increased by one unit use of assistive device,.
Surgical
The direct effect of the surgical factor on Ln (LOS) was 0.36 (p=0.001, 95% CI=.15 – .64), which is equal to 1.44 (95% CI=1.16 – 1.44) for LOS, indicating that the LOS is increased by1.44 times when the surgical factor is increased by one unit of severity of illness,
Post-surgical
The direct effect of the post-surgical factor on Ln (LOS) was −0.08 (p=0.001, 95%CI = −0.09 – −0.06), which is equal to −1.08(95% CI=1.10 – 1.06) indicating that the LOS is decreased slightly by 1.08 times when the post-surgical factor is increased by one unit of square-root of gait.
Standardized effects
Standardized effects of the latent factors (Figure 1) showed that the post-surgical factor was the most influential in explaining the variation in LOS (standardized estimates = −.50, 95% CI = − 0.58 – −0.41, p=0.001), followed by the surgical factor (standardized estimates = .21, 95% CI = .11 – .32, p=0.001). The pre-surgical factor was the least influential on the LOS (standardized estimates = .13, 95% CI = 0.01 – 0.23, p=0.03).
Variability in the LOS
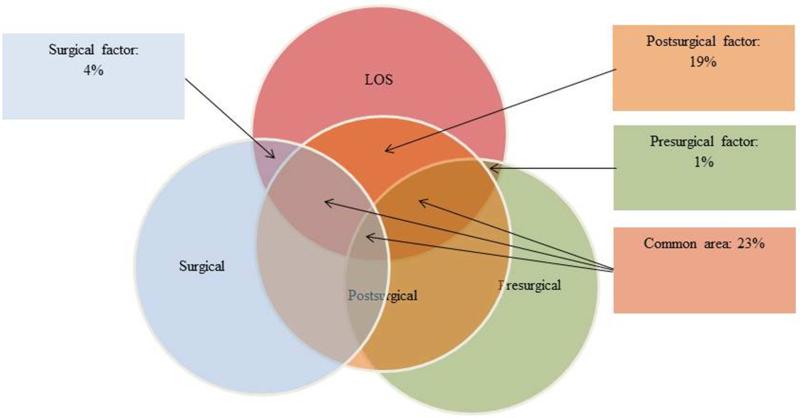
The three latent factors explained 47% of the variation in LOS (Figure 2). Variation partitioning showed that the post-surgical factor had by far the strongest independent effect on LOS, accounting for 19% of the explained variance. In comparison, independent pre-surgical (1%) and surgical (4%) effects were weak. The common area in Figure 2 indicates that 23% of the variation in LOS can be explained by at least two factors together; post-surgical and pre-surgical or post-surgical and surgical.
Figure 2.
Shared variability of the factors predicting the LOS. All factors predicted 47% of variability. Values presented are % of individual factors as well as shared effects. Independent variability of pre-surgical, surgical, and post-surgical factors is 1%, 4%, and 19% respectively. Shared variability by all three factors is 23%, as indicated by the common area.
Correlation and covariance
There was significant (p=0.001) correlation and covariance between the three latent variables (Table 4). There was a strong negative relationship between pre- and post-surgical factors (r= – .53), a moderate positive relationship between pre-surgical and surgical factors (r=0.38), and a less moderate negative relationship between surgical and post-surgical factors (r= −0.32). The post-surgical factor had a strong negative correlation with the pre-surgical factor and moderate negative correlation with the surgical factor. Meanwhile, the covariance between pre-surgical and surgical factors is almost independent.
Table 4.
Correlation and covariance estimates
| Correlation | Covariance | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | S.E. | CI | p | Estimate | S.E. | CI | p | |
| Presurgical with surgical | .38 | .09 | .21 - .56 | .001 | .08 | .02 | .03 - .12 | .001 |
| Presurgical with postsurgical | −.53 | .05 | −.62 - −.43 | .001 | −1.14 | .14 | −1.14 - −.86 | .001 |
| Surgical with postsurgical | −.32 | .06 | −.46 - −.21 | .002 | −.63 | .16 | −.94 - −.32 | .001 |
S.E: standard error
Discussion
The study sought to retrospectively identify surgical and non-surgical factors predictive of LOS following lumbar laminectomy and fusion. One of the important features of this study was that we analyzed correlation, covariance and shared variability among many factors that have not been considered in previous studies. Our 3-factor SEM explained 47% of the variation in LOS. Twenty three percent of the variation was shared between at least two out of the three factors, whereas 24% of the variation was independent of the three factors contributing to the LOS. Of the three factors studied, the post-surgical factor was the strongest predictor of LOS and explained 19% of the variation.
Many factors were associated with LOS but they were not strong predictors. Variables such as sex, race, living situation, type of home, hematocrit levels before surgery, number of comorbidities, type of surgery, diagnosis, total volume of fluid resuscitation, hemoglobin and hematocrit levels after surgery, and post-operative pain location were significantly correlated with LOS. However, these variables were dropped from the final SEM presumably due to their modest effect and collinearity with other variables. Our findings are similar to previous studies in which race and sex were shown to be either minimally correlated [6, 10, 12] or had modest significant effects on LOS [8]. Similarly, living situation and type of home have been shown to be correlated but not significant predictors of LOS [14, 39]. Although the type of surgery was strongly correlated with LOS, this variable was dropped from the model due to its shared correlation with other variables like ICU LOS, complications, illness severity, and walking distance.
Hematocrit level before surgery was strongly correlated with hemoglobin; by default, one of these factors was dropped from the model. Hematocrit and hemoglobin levels after surgery and total volume of fluid resuscitation were only correlated with LOS. They were not significant predictors in our study and in a previous LSS study [4]. In addition, these variables had collinearity effects amongst each other, as well as with pre-surgical hemoglobin levels. Comorbidities have been shown to be significant predictors of LOS in previous studies [8, 12]. However, this construct was dropped from our model. The types of comorbidities might influence LOS differently, like diabetes being the only comorbidity that significantly predicted LOS following a joint replacement surgery [40]. Therefore, the effect of different types of comorbidities would be useful to assess in future studies.
Our results show that as a patient's age increases and the pre-operative level of hemoglobin and PLOF decreases, the probability for that patient to stay longer in hospital increases. Pre-surgical factors have been extensively utilized to predict LSS outcomes in previous studies, yet in our study they explained a very limited percentage of variation in LOS following LSS. Pre-surgical factors alone would influence LOS but when studied in conjunction with post-surgical factors, their predictive value is less influential. However, inclusion of standardized tests such as functional questionnaires, psychosocial measures, and patients’ expectations could improve model testing and contribution of pre-surgical factors toward LOS.
Increased age is always linked with longer LOS due to a greater number of comorbidities and higher rate of complications following surgery. Age was associated with LOS in the majority of previous studies of LSS [41-43] and other orthopedic surgeries [10, 12, 14]. Severity of illness also affects the patient's progress toward recovery and requires a greater need for clinical supervision, and therefore, a longer stay at the hospital. Severity of illness is usually calculated based on age, number of comorbidities, and both primary and secondary diagnoses upon discharge. In our study, severity of illness had stronger correlation with number of comorbidities than age (see appendix B); this may explain why the number of comorbidities variable was dropped from the SEM, but age was not.
Although PLOF and use of assistive devices are parts of routine assessment for patients being admitted to hospital, they have rarely been used as predictors of LOS following LSS. Pre-operative use of walking aids was a significant predictor of LOS after total joint replacement surgeries [12]. PLOF has been used previously in only one LSS study, but it was combined with post-surgical dependency scores [6], which limited the predictive estimates of PLOF. Use of assistive devices and PLOF reflect patients’ functional ability before surgery. These factors are modifiable and could be improved by rehabilitation to optimize surgery outcomes. It has been shown that patients who received rehabilitation before and immediately after surgery had a significant reduction in LOS following LSS in comparison to subjects who received rehabilitation only after surgery [44]. Finally, pre-operative hemoglobin is a significant predictor in our study, which contradicts a previous finding in a similar LSS study that included patients with only revision surgeries [4]. Our sample included patients with both primary and revision surgeries. Improving PLOF especially in older adults should be considered prior to LSS to improve surgical outcomes. In addition, clinicians should consider strategies to reduce blood loss and maintain the hemoglobin level within the physiological limits through pharmacological interventions and perioperative blood transfusion [45]. Elevating hemoglobin may help improve surgery outcomes and ultimately reduce LOS.
Surgical complications are one of the most common features reported to affect the LOS in spine [46-48] and other surgeries [10, 11, 14]. In our study, the percentage of patients who had complications was relatively low compared to other studies. Smith et al., [49] reported that 7% of patients experienced surgical complications following lumbar decompression surgery. With recent advances in spine surgery, these figures are likely to decrease and result in reducing LOS.
Post-surgical variables, especially PT assessment variables are rarely used to predict LOS. Our results show that the post-surgical factor is a significant predictor and can uniquely explain 19% of the variation in predicting LOS. Our findings confirm the importance of PT assessment as a predictor of LOS relating to the post-surgical factor in this LOS SEM. Functional dependency score was reported to be a major predictor of LOS following LSS [6]. In our study, gait distance and balance were used as predictors of LOS. Ability to walk and walking distance have been reported to predict discharge destination following total hip replacement [19, 50] and following LSS [51], while balance, to our knowledge, was not reported before in similar studies. Clinicians should consider better post-operative pain management approaches and increased frequency of post-operative rehabilitation as possible strategies to modify post-surgical factors and facilitate patient's functional recovery to shorten LOS. Studies that investigated post-surgical variables have shown that patients needing less assistance during walking, bed mobility, and transfer (dependency score), and patients with improved balance scores had better function and shorter hospital LOS. These functional predictors for LOS could be used to guide accelerated rehabilitation programs after LSS. The accelerated programs have already been shown to be efficient in reducing LOS and improving surgery outcomes following similar orthopedic surgeries [52].
Limitations
One of the limitations of our study is that we did not study the effect of various payment methods on LOS. Patients treated under health maintenance organizations have significantly shorter times in the hospital than those treated under fee-for-service plans [53, 54]. It would be useful to study the impact of implementing different payment systems on LOS simultaneously with various factors affecting LOS. Also, the quality and type of community care (e.g. family assist, or home health) and types of comorbidities may influence the discharge planning and LOS. Generalizability of our prediction models would have some limitations: 1) this is a retrospective study with the inherent problems of missing data, 2) the assessment was conducted by many healthcare professionals and may not be consistent, 3) and the data was taken from one healthcare center; the difference in practice patterns between regions and health centers in the same region should be taken into consideration [2].
Conclusion
LOS is a complex and multifaceted occurrence with numerous measurable and intangible factors. Our study showed that pre-surgical, surgical, and post-surgical factors have shared variability in explaining LOS. The post-surgical factor constructed from PT assessment variables appeared to have the highest independent variability where other factors explained relatively little variability of LOS. Functional measures, pre- and post-surgery, were the important predictors of LOS and can be modified to improve surgical outcomes. The study findings may provide guidelines to improve surgical outcomes i.e. by improving patient's pre-surgical hemoglobin level and preand post-surgery functional status. It is highly recommended that prediction of LOS be reproduced in a prospective study and should include standardized assessment predictors to improve prediction accuracy.
Supplementary Material
Acknowledgements
Blinded
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
No duality of interest to declare.
References
- 1.Cherkin DC, Deyo RA, Loeser JD, Bush T, Waddell G. An international comparison of back surgery rates. Spine (Phila Pa 1976) 1994;19(11):1201–6. doi: 10.1097/00007632-199405310-00001. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States' trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976) 2006;31(23):2707–14. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan C, Catlin A, Smith C, Sensenig A. National health expenditures, 2002. Health Care Financ Rev. 2004;25(4):143–66. [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng F, Cammisa FP, Jr., Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine (Phila Pa 1976) 2002;27(8):818–24. doi: 10.1097/00007632-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Nahtomi-Shick O, Kostuik JP, Winters BD, Breder CD, Sieber AN, Sieber FE. Does intraoperative fluid management in spine surgery predict intensive care unit length of stay? J Clin Anesth. 2001;13(3):208–12. doi: 10.1016/s0952-8180(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 6.Sharma NK, Arnold PM, McMahon JK, Loyd L, Sabus CH, O'Connor BR. Acute Physical Therapy and Length of Hospital Stay Following Lumbar Discectomy and Lumbar Fusion: A Retrospective Analysis. JACPT. 2012;3(1):157–63. [Google Scholar]
- 7.Siemionow K, Pelton MA, Hoskins JA, Singh K. Predictive factors of hospital stay in patients undergoing minimally invasive transforaminal lumbar interbody fusion and instrumentation. Spine. 2012;37(24):2046–54. doi: 10.1097/BRS.0b013e31825c6688. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walid MS, Robinson EC, Robinson JS., Jr. Higher comorbidity rates in unemployed patients may significantly impact the cost of spine surgery. J Clin Neurosci. 2011;18(5):640–4. doi: 10.1016/j.jocn.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Arnold PM, Rice LR, Anderson KK, McMahon JK, Connelly LM, Norvell DC. Factors affecting hospital length of stay following anterior cervical discectomy and fusion. Evid Based Spine Care J. 2011;2(3):11–8. doi: 10.1055/s-0030-1267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BuSaba NY, Schaumberg DA. Predictors of prolonged length of stay after major elective head and neck surgery. Laryngoscope. 2007;117(10):1756–63. doi: 10.1097/MLG.0b013e3180de4d85. [DOI] [PubMed] [Google Scholar]
- 12.Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168–73. doi: 10.1080/17453670710014941. [DOI] [PubMed] [Google Scholar]
- 13.Schneider M, Kawahara I, Ballantyne G, et al. Predictive factors influencing fast track rehabilitation following primary total hip and knee arthroplasty. Arch Orthop Trauma Surg. 2009;129(12):1585–91. doi: 10.1007/s00402-009-0825-9. [DOI] [PubMed] [Google Scholar]
- 14.Epps CD. Length stay, discharge disposition, and hospital charge predictors. AORN J. 2004;79(5):975–97. doi: 10.1016/s0001-2092(06)60729-1. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber JB, Nora A, Stagec FK, Barlowb EA, Kinga J. Reporting Structural Equation Modeling and Confirmatory Factor Analysis Results: A Review. J Educ Res. 2006;99(6):323–38. [Google Scholar]
- 16.MacCallum RC, Austin JT. Applications of Structural Equation Modeling in Psychological Research. Annual Review of Psychology. 2000;51(1):201–26. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Waitman LR, Warren JJ, Manos EL, Connolly DW. Expressing observations from electronic medical record flowsheets in an i2b2 based clinical data repository to support research and quality improvement. AMIA Annu Symp Proc. 2011;2011:1454–63. [PMC free article] [PubMed] [Google Scholar]
- 18.DeBerard MS, LaCaille RA, Spielmans G, Colledge A, Parlin MA. Outcomes and presurgery correlates of lumbar discectomy in Utah Workers' Compensation patients. Spine J. 2009;9(3):193–203. doi: 10.1016/j.spinee.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.den Boer JJ, Oostendorp RA, Beems T, Munneke M, Oerlemans M, Evers AW. A systematic review of bio-psychosocial risk factors for an unfavourable outcome after lumbar disc surgery. Eur Spine J. 2006;15(5):527–36. doi: 10.1007/s00586-005-0910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaetani P, Aimar E, Panella L, Debernardi A, Tancioni F, Rodriguez y Baena R. Surgery for herniated lumbar disc disease: factors influencing outcome measures. An analysis of 403 cases. Funct Neurol. 2004;19(1):43–9. [PubMed] [Google Scholar]
- 21.Harvey RE, Kallmes DF. Discharge disposition following vertebroplasty. AJNR Am J Neuroradiol. 2011;32(9):1614–6. doi: 10.3174/ajnr.A2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaCaille RA, DeBerard MS, Masters KS, Colledge AL, Bacon W. Presurgical biopsychosocial factors predict multidimensional patient: outcomes of interbody cage lumbar fusion. Spine J. 2005;5(1):71–8. doi: 10.1016/j.spinee.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Mannion AF, Elfering A, Staerkle R, et al. Predictors of multidimensional outcome after spinal surgery. Eur Spine J. 2007;16(6):777–86. doi: 10.1007/s00586-006-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nygaard OP, Kloster R, Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg. 2000;92(2 Suppl):131–4. doi: 10.3171/spi.2000.92.2.0131. [DOI] [PubMed] [Google Scholar]
- 25.Trief PM. A prospective study of psychological predictors of lumbar surgery outcome. Spine (Philadelphia, Pa 1976) 2000;25(20):2616–21. doi: 10.1097/00007632-200010150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Trief PM, Ploutz-Snyder R, Fredrickson BE. Emotional health predicts pain and function after fusion: a prospective multicenter study. Spine (Phila Pa 1976) 2006;31(7):823–30. doi: 10.1097/01.brs.0000206362.03950.5b. [DOI] [PubMed] [Google Scholar]
- 27.Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85-A(1):102–8. [PubMed] [Google Scholar]
- 28.de Pablo P, Losina E, Phillips CB, et al. Determinants of discharge destination following elective total hip replacement. Arthritis Rheum. 2004;51(6):1009–17. doi: 10.1002/art.20818. [DOI] [PubMed] [Google Scholar]
- 29.DeJong G, Hsieh CH, Putman K, Smout RJ, Horn SD, Tian W. Physical therapy activities in stroke, knee arthroplasty, and traumatic brain injury rehabilitation: their variation, similarities, and association with functional outcomes. Phys Ther. 2011;91(12):1826–37. doi: 10.2522/ptj.20100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallinson TR. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011;92(5):712–20. doi: 10.1016/j.apmr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton B GC, Sherwin F, Zielezny M, Tashman J. A uniform national data system for medical rehabilitation. In: Fuhrer MJ, editor. Rehabilitation outcomes: analysis and measurement. 1st ed. Paul H. Brookes; Baltimore: 1987. [Google Scholar]
- 32.Kluding P, Swafford B, Cagle P, Gajewski B. Reliability, responsiveness, and validity of the Kansas University Standing Balance Scale. J Geriatr Phys Ther. 2006;29(3):93–9. doi: 10.1519/00139143-200612000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Iezzoni LI, Ash AS, Shwartz M, Daley J, Hughes JS, Mackiernan YD. Predicting who dies depends on how severity is measured: implications for evaluating patient outcomes. Ann Intern Med. 1995;123(10):763–70. doi: 10.7326/0003-4819-123-10-199511150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Laws C, Colon D. Comorbidities in Workers Compensation. NCII Research Brief [serial on the Internet] 2012 Available from: https://www.ncci.com/documents/Research-Brief-Comorbidities-in-Workers-Compensation-2012.pdf.
- 35.Barnett V, Lewis T. Outliers in Statistical Data. 2nd ed. JohnWiley; New York: 1994. [Google Scholar]
- 36.Mardia KV. Measures of Multivariate Skewness and Kurtosis with Applications. Biometrika. 1970;57(3):519–30. [Google Scholar]
- 37.Bollen K, Curran P. 1st ed. John Wiley & Sons; Hoboken, NJ: 2005. Latent Curve Models: A Structural Equation Perspective. [Google Scholar]
- 38.Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 39.Kelly MH, Ackerman RM. Total joint arthroplasty: a comparison of postacute settings on patient functional outcomes. Orthop Nurs. 1999;18(5):75–84. [PubMed] [Google Scholar]
- 40.Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186–90. doi: 10.1016/s0883-5403(98)90097-3. [DOI] [PubMed] [Google Scholar]
- 41.Hauck K, Zhao X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med Care. 2011;49(12):1068–75. doi: 10.1097/MLR.0b013e31822efb09. [DOI] [PubMed] [Google Scholar]
- 42.Neatherlin JS, Brillhart B, Henry JJ. Factors determining length of hospitalization for patients having laminectomy surgery. J Neurosci Nurs. 1988;20(1):39–41. doi: 10.1097/01376517-198802000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Jo DJ, Jun JK, Kim KT, Kim SM. Lumbar Interbody Fusion Outcomes in Degenerative Lumbar Disease : Comparison of Results between Patients Over and Under 65 Years of Age. J Korean Neurosurg Soc. 2010;48(5):412–8. doi: 10.3340/jkns.2010.48.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen PR, Jorgensen LD, Dahl B, Pedersen T, Tonnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24(2):137–48. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 45.Diamond PT, Conaway MR, Mody SH, Bhirangi K. Influence of hemoglobin levels on inpatient rehabilitation outcomes after total knee arthroplasty. J Arthroplasty. 2006;21(5):636–41. doi: 10.1016/j.arth.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Clarke A, Rosen R. Length of stay. How short should hospital care be? Eur J Public Health. 2001;11(2):166–70. doi: 10.1093/eurpub/11.2.166. [DOI] [PubMed] [Google Scholar]
- 47.Kilincer C, Steinmetz MP, Sohn MJ, Benzel EC, Bingaman W. Effects of age on the perioperative characteristics and short-term outcome of posterior lumbar fusion surgery. J Neurosurg Spine. 2005;3(1):34–9. doi: 10.3171/spi.2005.3.1.0034. [DOI] [PubMed] [Google Scholar]
- 48.Deyo RA, Cherkin DC, Loeser JD, Bigos SJ, Ciol MA. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis, and procedure. J Bone Joint Surg Am. 1992;74(4):536–43. [PubMed] [Google Scholar]
- 49.Smith JS, Fu KM, Polly DW, Jr., et al. Complication rates of three common spine procedures and rates of thromboembolism following spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2010;35(24):2140–9. doi: 10.1097/BRS.0b013e3181cbc8e7. [DOI] [PubMed] [Google Scholar]
- 50.Félix I, Fritzsche K. Determining predictors that affect discharge destination from the acute care setting following elective total hip arthroplasty. Top Geriatr Rehabil. 2004;20(4):316–17. [Google Scholar]
- 51.Kanaan SF, Yeh H-W, Waitman RL, Burton DC, Arnold PM, Sharma NK. Predicting Discharge Placement and Health Care Needs After Lumbar Spine Laminectomy. J Allied Health. 2014;43(2):88–97. [PMC free article] [PubMed] [Google Scholar]
- 52.den Hertog A, Gliesche K, Timm J, Muhlbauer B, Zebrowski S. Pathway-controlled fast-track rehabilitation after total knee arthroplasty: a randomized prospective clinical study evaluating the recovery pattern, drug consumption, and length of stay. Arch Orthop Trauma Surg. 2012;132(8):1153–63. doi: 10.1007/s00402-012-1528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke A. Why are we trying to reduce length of stay? Evaluation of the costs and benefits of reducing time in hospital must start from the objectives that govern change. Qual Health Care. 1996;5(3):172–9. doi: 10.1136/qshc.5.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradbury RC. Comparing hospital length of stay in independent practice association HMOs and traditional insurance programs. Inquiry (Chicago) 1991;28(1):87–93. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.