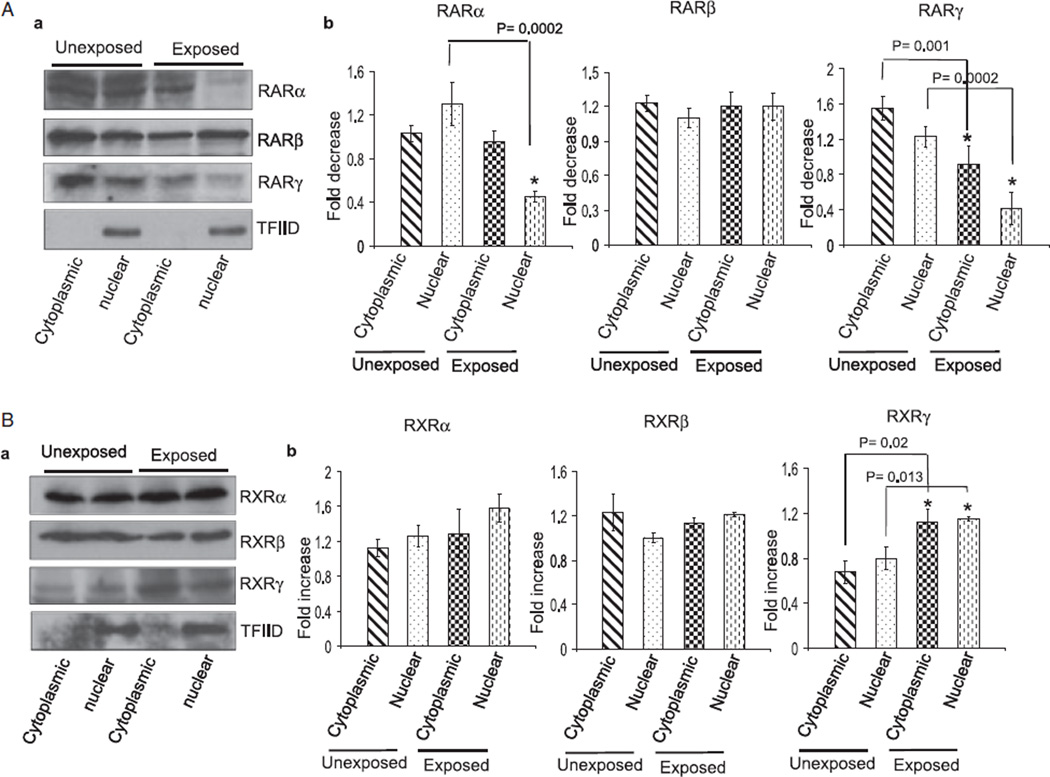
Fig. 6.
Ethanol alters the cytoplasmic and nuclear distribution of RA receptors in CGNs. The isolation and primary culture of CGNs from ethanol-exposed and unexposed pups was performed in the presence and absence of ethanol as noted in Fig. 5. Cytoplasmic and nuclear proteins were isolated from CGNs of unexposed and ethanol-exposed pups as noted in the Methods section. An equal amount of proteins was separated by SDS–PAGE and transferred to a nitrocellulose membrane for Western blotting using anti-RA receptor antibodies. (A) Western blot analysis of cytoplasmic and nuclear proteins using anti-RARα, β, and γ antibodies. Membranes were blotted with a TFIID antibody to check the purity of nuclear proteins. Total cell lysates were run in a parallel set to monitor the level of actin in each sample (data not presented). The amounts of RARα, β, and γ in the cytoplasmic and nuclear fractions were calculated by scanning densitometric analysis of respective bands. The ratio of the scanned density of the band belonging to the individual receptor with that of actin is represented as fold increase or decrease. Results are mean of ± SD of three separate experiments. (B) Similar methods were used to analyze the ethanol-induced changes in the cytoplasmic and nuclear levels of RXRs.