Table 2.
Synthesis of diethyl 4-alkyl-2,2-oxetane dicarboxylates. 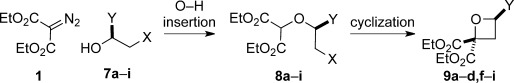
| Entry | X | Y | Yield8 [%][a] | Yield9 [%][b] | |
|---|---|---|---|---|---|
| 1 | Br | CH2OBn | a | 67 | 89 |
| 2 | Br | CH2OPh | b | 92 | 65 |
| 3 | Br | CH2Br | c | 51[c] | 81 |
| 4 | Br | CH2Cl | d | 80 | 45/7 (Y=Cl/Br, 9 d/9 c) |
| 5 | Cl | CH2Cl | e | 86 | 77 (9 d) |
| 6 | Cl | CH2OiPr | f | 97 | 75 |
| 7 | Cl | CH2OTBS | g | 65 | 71 |
| 8 | Br | CF3 | h | 28[d] | 43 |
| 9 | Br | CH3[e] | i | 98[f] | 82[g] |
O=H insertion conditions: 7 (1.0–3.0 mmol), 1 (1.5 equiv), [Rh2(OAc)4] (0.5 mol %), PhH, 0.1 m, 80 °C.
Cyclization conditions: 8 (0.4–1.0 mmol), NaH (1.2 equiv), DMF, 0.025 m, 25 °C, 16 h.
Heated at 80 °C for 3 d.
Yield over two steps from 3-bromo-1,1,1-trifluoroacetone.
From technical grade 1-bromo-2-propanol (7 i) containing 20 wt % 2-bromo-1-propanol.
Mixture of regioisomers (4:1).
Mixture of regioisomers (4-Me/3-Me oxetanes 5.4:1). Bn=benzyl, TBS=tert-butyldimethylsilyl.
