Table 3.
C=H hetero-difunctionalization of BQ. 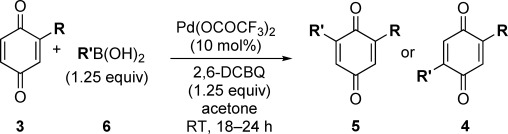
| Entry | R | R′ | Yield5[%][a] | Yield4[%][a] |
|---|---|---|---|---|
| 1 | p-MeO-C6H4 | p-HO-C6H4 | 73 (5 j) | <5 (4 j) |
| 2 | m,p-(MeO)2-C6H3 | p-HO-C6H4 | 71 (5 k) | 10 (4 k) |
| 3 | m,p-(MeO)2-C6H3 | p-MeO-C6H4 | 65 (5 l) | 21 (4 l) |
| 4 | p-HO-C6H4 | o-MeO-C6H4 | 50 (5 m) | 41 (4 m) |
| 5 | m,p-(MeO)2-C6H3 | p-EtO2C-C6H4 | 44 (5 n) | 26 (4 n) |
| 6 | p-EtO2C-C6H4 | m,p-(MeO)2-C6H3 | 48 (5 n) | 16 (4 n) |
| 7[d] | p-EtO2C-C6H4 | p-F3C-C6H4 | <5 (5 o) | 47 (4 o) |
| 8[b] | N-Boc-pyrrole-2 | 3-thiophene | trace | 74 (4 p) |
| 9[b–d] | m-O2N-C6H4 | 3-thiophene | trace | 42 (4 q) |
| 10[d] | p-EtO2C-C6H4 | 3-thiophene | trace | 34 (4 r) |
| 11 | m,p-(MeO)2-C6H3 | cyclohexyl | –[e] | – |
Yields of isolated products.
2.5 equiv of boronic acid 6 used.
[c] Treated with FeCl3 at the end of reaction.
Product only moderately stable.
Complex mixture of products.
