Abstract
The prognosis and prediction of axillary lymph node (ALN) metastases in breast cancer is traditionally based upon the biomarkers status of the primary tumor. Some retrospective studies showed significant discordance in receptor expression between primary and metastatic tumors. We aim to prospectively assess the incidence of discordant biomarkers status in primary tumor and ALN metastases and to evaluate the role of ALN biopsies for the reassessment of receptor status. Tissue arrays were constructed from 54 breast cancer patients with ALN metastases diagnosed. Arrays were immuno-stained to compare protein expression of four biomarkers including estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67 by immunohistochemistry. The kappa value of consistency in the primary tumor and the metastatic lymph nodes were 0.465 for ER, 0.445 for PR, and 0.706 for HER2. Good consistency was shown for Ki67 expression in primary and metastases regions with T test. No significant difference is existed between primary tumor and ALN metastases. It is concluded that the good consistency is present for ER, PR, HER2 and Ki67 between the primary tumor and the metastatic lymph nodes, suggesting that ER, PR, HER2, or Ki67 status in primary tumors could reflect their status in ALN metastases.
Keywords: Estrogen receptor, progesterone receptor, Ki67, HER2, breast cancer, immunohistochemistry
Introduction
Breast cancer is still one of the leading causes of malignancy death in women although there has been a sustained decline in mortality rates over the last decades. The incremental application of increasingly effective adjuvant medical treatments is one of the major factors for this development, despite an increasing incidence of breast cancer [1].
Classical histopathologic features indicate patient prognosis include tumor size, histological subtype and grade, lymph node metastases, and lymphovascular invasion, which are derived from careful histological analysis of primary breast cancer samples. These histopathologic factors indicate tumor stages with major prognostic value. Many novel biomarkers of the primary tumor have been reported for the better understanding of female breast cancer tumorgenesis, disease progression, treatment guidance, prognostic and predictive purposes. The evaluation of biomarkers is usually performed by analyzing the primary tumor tissues but this approach does not take into account potential discrepancies between primary tumor and secondary lesions.
The established traditional molecular biomarkers such as estrogen receptor (ER) and progesterone receptor (PR) are not only the classical clinical prognostic factors, but also played a significant role in the selection of patients benefiting from endocrine therapy [1]. More recently, the human epidermal growth factor receptor2 (HER2) has been validated to be not only a prognostic factor, but also a predictor of response to HER2 targeting therapy. At present, the therapeutic regimen chosen generally depends on subtype groups that are determined by immunohistochemical pattern of expression of ER, PR and HER2 [2]. In addition, another biomarker, Ki67, is a cell proliferation marker, which is expressed in the nuclei of cells in G1, S and M cell cycle phases. Ki67 has recently emerged as an important marker due to several applications in neoadjuvant therapy in addition to its moderate prognostic value. It is generally accepted that the Ki67 labeling index is a prognostic factor, and there is a tendency for Ki67 labeling index to decrease after chemotherapy [3-5] and that a larger decrease of Ki67 is correlated with better responsiveness to chemotherapy [4,6]. However, only few studies are performed on comparing molecular biomarkers status of primary tumor and axillary lymph node (ALN) metastases in the same patient.
The aim of this study is to examine and compare expressions of ER, PR, HER2, and Ki67 between primary tumor and ALN metastases of female breast cancer patients. All cancer tissues are from archived tumor blocks obtained from Qilu Hospital of Shandong University.
Materials and methods
Patient clinical data
Fifty four cases of breast cancer identified over 7 months were obtained from the in-patient department of the Qilu Hospital of Shandong University. All of the 54 cases of breast cancer are female, aged between 29 and 79 years old (median =47 years). All of the cases were diagnosed as breast cancer with ALN metastases by pathological examination. Among them, they are classified into invasive ductal carcinoma (49 cases), invasive lobular carcinoma (5 cases) by postoperative pathological diagnosis. Twenty four patients had preoperative neoadjuvant chemotherapy while 30 patients did not. All the patients were recorded as alive at their last known follow-up date.
Histology and immunohistochemistry
Tumor blocks were requested from Qilu Hospital of Shandong University for immunohistochemical staining for ER and PR, as well as recently described breast cancer prognostic markers such as HER2-Neu (DAKO), and Ki67 (MiB1-Innovex, Inc., Parsippany, NJ), using established antigen retrieval method. Pathology slides were reviewed by one pathologist for nuclear grading, classification of tumors, and evaluation of the immunohistochemical stains. Patient medical records and tissue blocks contained original identification numbers and therefore warranted the process of institutional review for use of human records and tissue; the study was approved by the institutional review board at Shandong University, PRC.
For immunohistochemistry, 4 µm sections were deparaffinized in xylene for 30 min and rinsed in 100%, 96% and 70% ethanol. Endogenous peroxidase was blocked by incubation in 3% H2O2 in methanol for 10 min, followed by rinsing in phosphate buffered saline (PBS). Sections were then subjected to antigen retrieval by immersion in citrate buffer (pH 6.0) preheated to 99°C for 40 min.
The sections were incubated overnight with the monoclonal primary antibodies against ER, PR, HER2 and Ki67 (ZSGB-BIO: series NO: ZA-0102, ZA-0255, ZA-0023, ZA-0502; 50 µl per section) at 4°C. After complete washes in PBS, sections were incubated with biotinylated secondary antibodies (50 µl per section) for 30 min at room temperature. The antigen-antibody immunoreaction was revealed with 3,3’-diaminobenzidine as the chromogen. The slides were counterstained with hematoxylin, and embedded in neutral resin. Appropriate positive controls from female breast tumors were used for each antibody. Negative controls were incubated with PBS, omitting primary antibodies.
Immunohistochemical scoring
Only nuclear staining was considered for the immunoreactivity of ER, PR and Ki67. Cytoplasmic staining was ignored. Immunostaining frequency of the tumor cells for ER-ir and PR-ir was scored subjectively on a scale of ‘-’ to ‘+++’ (-, 0% positive tumor cells; +, 0 to 25%; ++, 26% to 50%; +++, more than 50%). We classified the tumor as positive with a score of more than ‘+’ (immunohistochemistry) for ER and PR. The scoring of the immunoreactivity of HER2 was distinguishing between no staining (-), weakly (+), moderate (++), and strong membrane staining (+++). Cytoplasmic staining was ignored. HER2 was classified as positive with a score of +++ (uniform, intensive membrane staining of more than 30% of invasive tumor cells) and negative with an IHC staining of 0, +. Besides, some patients bearing HER2 ++ were retested with fluorescence in situ hybridization (FISH), and were classified to be positive or negative according to the results of FISH. The Ki-67 index was obtained by the percentage of tumor cells that were labeled at nuclei by Ki-67.
Statistical analysis
All of the data were analyzed by the software SPSS12.0. The consistency test was carried out for ER, PR, and HER2 between primary tumor and ALN metastases. Ki-67 was converted as the numeric variable.
Results
This study identified the expression of ER, PR, HER2 and Ki67 in the primary tumor and ALN metastases from 54 breast cancer patients by using immunohistochemistry.
According to Tables 1 and 2, the ER-ir positive cases in primary tumor accounts for 63% (n=34) while that in ALN metastases accounts for 42.6% (n=23). Consistency (Kappa =0.465, 0.363) was shown for ER expression in primary and metastases regions by consistency test (see Table 1). Discordance for ER was 27.8% (n=15) while the concordance rate was 72.2% (n=39). Among these, 24.1% (n=13) patients had ER-positive primary tumor but ER-negative metastasis and 3.7% (n=2) had ER-negative primary but ER-positive metastasis.
Table 1.
Expression and consistency of biomarkers in primary tumor and ALN metastases (n=54)
| ALN metastases | Kappa | T | P Value | ||
|---|---|---|---|---|---|
|
| |||||
| positive | negative | ||||
| ER | 0.465 | 3.715 | <0.01 | ||
| positive | 21 | 13 | |||
| negative | 2 | 18 | |||
| PR | 0.445 | 3.521 | <0.01 | ||
| positive | 45 | 1 | |||
| negative | 5 | 3 | |||
| HER2 | 0.706 | 5.27 | <0.01 | ||
| positive | 8 | 4 | |||
| negative | 1 | 41 | |||
Note. ALN= axillary lymph node, ER= estrogen receptor, PR= progesterone receptor.
Table 2.
Expression of different grade and consistency of ER and PR in primary tumor and ALN metastases (n=54)
| ALN metastases | P Value | ||||
|---|---|---|---|---|---|
|
| |||||
| - | ﹢ | ﹢﹢ | ﹢﹢﹢ | ||
| ER | <0.01 | ||||
| - | 18 | 0 | 2 | 0 | |
| ﹢ | 9 | 0 | 0 | 1 | |
| ﹢﹢ | 4 | 2 | 5 | 4 | |
| ﹢﹢﹢ | 0 | 0 | 2 | 7 | |
| PR | <0.01 | ||||
| - | 3 | 1 | 4 | 0 | |
| ﹢ | 1 | 6 | 7 | 3 | |
| ﹢﹢ | 0 | 2 | 11 | 7 | |
| ﹢﹢﹢ | 0 | 0 | 1 | 8 | |
Note. ER = estrogen receptor, PR = progesterone receptor, ALN = axillary lymph node.
In Tables 1 and 2, the PR-ir positive cases in primary region accounts for 84.3% (n=46) while that in metastases region accounts for 87% (50). Consistency (Kappa=0.445, 0.334) was shown for PR expression in primary and metastases regions by consistency test (see Table 1). Discordance for PR was 11.1% (n=6) while the concordance rate was 88.9% (n=48). Among these, 1.9% (n=1) patients had PR-positive primary tumor but PR-negative metastasis and 9.2% (n=5) had PR-negative primary but PR-positive metastasis.
HER2 positive cases in primary region accounts for 22.2% (n=12) while that in metastases region accounts for 16.7% (n=9). Good consistency (Kappa >0.7) was shown for HER2 expression in primary and metastases regions by consistency test (see Table 1). Discordance for HER2 was 9.3% (n=5) while the concordance rate was 90.7% (n=49). Among these, 7.4% (n=4) patients had HER2-positive primary tumor but HER2-negative metastasis and 1.9% (n=1) had HER2-negative primary but HER2-positive metastasis.
Ki-67 was converted as the numeric variable and present with normal distribution. Thus, we carried out the paired T test for two sets of data and the results are shown in Figure 1. The T test showed there is no significant difference for positive case in primary and metastases regions (P>0.05). Good consistency was shown for Ki67 expression in primary and metastases regions.
Figure 1.
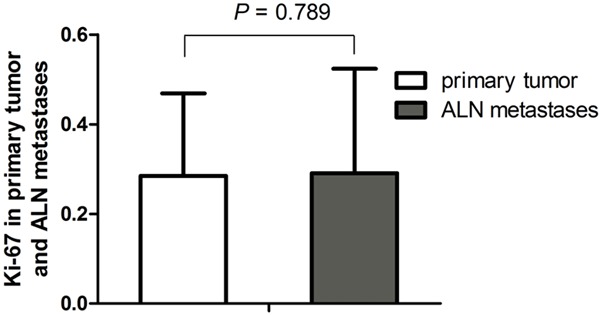
Ki67 level in primary tumor and ALN metastases. No statistically significant difference in the level of Ki67 (P=0.789) between the two groups.
Discussion
In many cases, it is presumed that the primary tumor has the same hormone sensitivity with that of ALN-metastases. Recently, it is questioned in view of increasing evidence of discordance between the receptor status of breast cancer primary tumors and their asynchronous metastases. Only 46% concordant rate was reported for ER and PR in the primary tumor and later in metastatic disease [7]. In one retrospective study of 80 patients the ER discordance rate was 21% and the PR rate was 37%, while there was no discordance for HER2 [8]. Another retrospective study of 200 patients found the ER discordance rate was 30% and PR rate was 39.3% [9]. In the present study, we found discordance between primary and metastatic ER, PR and HER2 were noted as 19.1%, 11.8% and 14.8% respectively. This study, albeit with a small sample size, has prospectively shown lower rates of discordance of ER and PR status between primary breast tumors and ALN-metastases to those in other published studies. Furthermore, our high concordance rates regarding ER (72.2%), PR (88.9%), and HER2 (90.7%) status between the primary tumor and the ALN-metastases are highly consistent with previous studies [10,11]. Previous works reported a more important variation for HER2 testing compared with recent data and this finding may be limited to bone metastases [12].
Our study showed HER2 overexpression in the primary tumor (22.2%) and the ALN-metastases (16.7%) without significant difference. Among them, 3 cases have low expression or negative in the primary tumor but overexpression in the ALN-metastases, and another 5 cases have the opposite occasion. It is good consistency for HER2 expressions between primary and metastases regions. Previous study reported that 15% of overexpression and amplification of HER2 was detected in primary breast cancers, and this group of patients benefit significantly from anti-HER2 therapies, suggesting HER2 status should be assessed in every diagnosed case of breast cancer [13,14].
Previous study reported the median Ki67 expression in primary and metastatic tumors was 20% and 15%, respectively [15]. We compared primary tumors with their corresponding metastatic lesions, and found that there were no significant differences in the expression of Ki67 between them, which is same with studies carried out by Kareem Tawfik, et al. [15], but different from studies have found greater expression of Ki67 in metastatic tumors compared with primary tumors [16-18]. That Ki67 expression is useful in metastatic tumors but not in primary tumors has been reported [16]. Interestingly, it was demonstrated that a high Ki67 in ALN but not in breast is significantly associated with shorter patient survival, suggesting that patients with higher Ki67 level in LN metastases might require more aggressive therapy and closer clinical monitoring of their disease. Therefore, further studies are recommended to further investigate the heterogeneity of both stem and tumor cells in primary and metastatic tumors to design therapies that are tailored to target the specific clones. Due to the small number of cases examined in this study, the reliability of our results is limited, and further study with more clinical cases is necessary to confirm that the difference of Ki67 between primary and metastases regions should be assessed for prediction of the tumor radiotherapy sensitivity.
In conclusion, we demonstrated there is no significant difference of ER, PR, HER2 and Ki67 status between primary tumor and ALN-metastases, suggesting that evaluation of ER, PR, HER2, or Ki67 status in either primary tumors or ALN-metastases has equal value for the development and appropriate use of anti-cancer agents, and ER, PR, HER2, or Ki67 status in primary tumors could reflect prognosis and prediction of ALN metastases.
Acknowledgements
It is supported by the Shandong Provincial Nature Funds (ZR2009CM007, Q2008C08).
Disclosure of conflict of interest
None.
References
- 1.Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17:R245–262. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ 10th St. Gallen conference. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer H, Gall C, Vogel U, Hornung R, Wallwiener D, Solomayer E, Fehm T. Changes in tumour biological markers during primary systemic chemotherapy (PST) Anticancer Res. 2008;28:1797–1804. [PubMed] [Google Scholar]
- 4.Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, Wright D, Allen SA, Dove J, Wilson GD. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92:147–155. doi: 10.1038/sj.bjc.6602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005;446:489–496. doi: 10.1007/s00428-005-1244-0. [DOI] [PubMed] [Google Scholar]
- 6.Burcombe R, Wilson GD, Dowsett M, Khan I, Richman PI, Daley F, Detre S, Makris A. Evaluation of Ki-67 proliferation and apoptotic index before, during and after neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Res. 2006;8:R31. doi: 10.1186/bcr1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amir E, Ooi WS, Simmons C, Kahn H, Christakis M, Popovic S, Kalina M, Chesney A, Singh G, Clemons M. Discordance between receptor status in primary and metastatic breast cancer: an exploratory study of bone and bone marrow biopsies. Clin Oncol (R Coll Radiol) 2008;20:763–768. doi: 10.1016/j.clon.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, Miller N, Andrulis IL, Brenner DM, Clemons MJ. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–1562. [PubMed] [Google Scholar]
- 9.Lower EE, Glass EL, Bradley DA, Blau R, Heffelfinger S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90:65–70. doi: 10.1007/s10549-004-2756-z. [DOI] [PubMed] [Google Scholar]
- 10.Nedergaard L, Haerslev T, Jacobsen GK. Immunohistochemical study of estrogen receptors in primary breast carcinomas and their lymph node metastases including comparison of two monoclonal antibodies. APMIS. 1995;103:20–24. doi: 10.1111/j.1699-0463.1995.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 11.Bassarova AV, Nesland JM, Sedloev T, Lilleby W, Hristova SL, Trifonov DY, Torlakovic E. Simultaneous bilateral breast carcinomas: a category with frequent coexpression of HER-2 and ER-alpha, high Ki-67 and bcl-2, and low p53. Int J Surg Pathol. 2005;13:239–246. doi: 10.1177/106689690501300302. [DOI] [PubMed] [Google Scholar]
- 12.Lorincz T, Toth J, Badalian G, Timar J, Szendroi M. HER-2/neu genotype of breast cancer may change in bone metastasis. Pathol Oncol Res. 2006;12:149–152. doi: 10.1007/BF02893361. [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ, team HS. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 15.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2013;44:39–46. doi: 10.1016/j.humpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Park D, Karesen R, Noren T, Sauer T. Ki-67 expression in primary breast carcinomas and their axillary lymph node metastases: clinical implications. Virchows Arch. 2007;451:11–18. doi: 10.1007/s00428-007-0435-2. [DOI] [PubMed] [Google Scholar]
- 17.Buxant F, Anaf V, Simon P, Fayt I, Noel JC. Ki-67 immunostaining activity is higher in positive axillary lymph nodes than in the primary breast tumor. Breast Cancer Res Treat. 2002;75:1–3. doi: 10.1023/a:1016504129183. [DOI] [PubMed] [Google Scholar]
- 18.Tokes AM, Szasz AM, Geszti F, Lukacs LV, Kenessey I, Turanyi E, Meggyeshazi N, Molnar IA, Fillinger J, Soltesz I, Balint K, Hanzely Z, Arato G, Szendroi M, Kulka J. Expression of proliferation markers Ki67, cyclin A, geminin and aurora-kinase A in primary breast carcinomas and corresponding distant metastases. J Clin Pathol. 2015;68:274–82. doi: 10.1136/jclinpath-2014-202607. [DOI] [PubMed] [Google Scholar]


