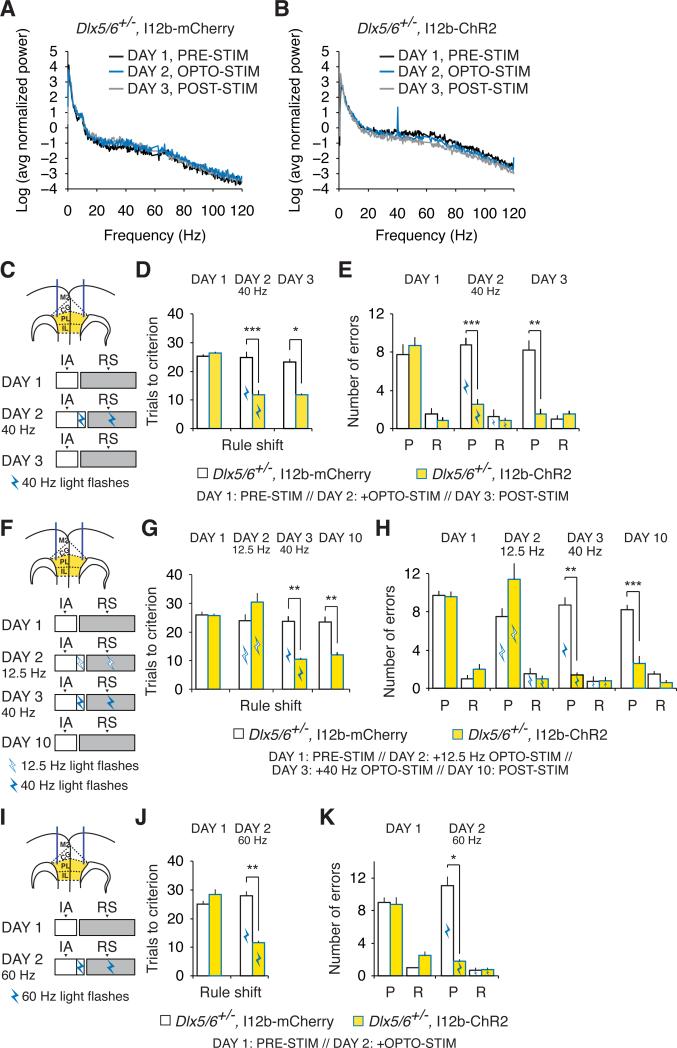
Figure 7. Optogenetic augmentation of interneuron function rescues cognitive flexibility in adult Dlx5/6+/− mice.
(A) Log transform of the normalized power spectrum for prefrontal EEG recordings from an adult Dlx5/6+/− mouse injected with AAV-I12b-mCherry in the mPFC during the first ten trials of the rule shift portion of the task in the absence of light stimulation on Day 1, in the presence of optogenetic stimulation (40 Hz, 5 msec) on Day 2, and in the absence of light stimulation on Day 3. For this plot, the power spectrum from each mouse was normalized by the sum of all values from 0-200 Hz (excluding 58-62 Hz).
(B) Similar to (A) but shows power spectrum of prefrontal EEG recordings from an adult Dlx5/6+/− mouse injected with AAV-I12b-ChR2 in the mPFC.
(C) Schematic illustrating the design of experiments using 40 Hz optogenetic stimulation of mPFC interneurons during rule shifting. Dual fiber-optic cannulae were implanted bilaterally in the mPFC of Dlx5/6+/− mice. AAV (yellow) was also injected into the PFC bilaterally to drive expression of either ChR2-eYFP or mCherry in interneurons using the I12b enhancer. On Day 1, animals performed the initial association (IA) and rule shift (RS) in the absence of optogenetic stimulation. On Day 2, after the 80% correct criterion was reached in the IA, optogenetic stimulation was switched on, and continued during three additional IA trials and the subsequent RS. On Day 3, animals again performed the IA and RS in the absence of optogenetic stimulation. Optogenetic stimulation = blue light flashes at 40 Hz.
(D) Rule shift performance does not differ between Dlx5/6+/− mice that express ChR2 or mCherry in interneurons on Day 1, prior to optogenetic stimulation. However, 40 Hz stimulation on Day 2 selectively normalizes rule shifting in ChR2-expressing Dlx5/6+/− mice. Dlx5/6+/− mice that express ChR2 maintain improved cognitive flexibility on Day 3, even without additional light stimulation.
(E) The improved rule-shifting performance in (D) is accompanied by a decrease in perseverative errors (P).
(F) Similar to (C), the experimental design for experiments in which animals perform the IA and RS in the absence of optogenetic stimulation on Day 1, then receive 12.5 Hz optogenetic stimulation of PFC interneurons on Day 2, and 40 Hz optogenetic stimulation on Day 3.
(G and H) Unlike 40 Hz stimulation, the 12.5 Hz pattern of stimulation depicted in (F) fails to improve either the number of trials required to learn the rule shift (G). Dlx5/6+/− mice that express ChR2 maintain improved cognitive flexibility on Day 10 (i.e., 7 days after 40 Hz stimulation), even in the absence of additional light stimulation (G).
(I-K) 60 Hz optogenetic stimulation also improves learning of a rule shift and reduces preservative errors in ChR2-expressing Dlx5/6+/− mice.
All data show means ± SEM and are analyzed using two-tailed Student's t-tests or repeated measures ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001.
See also Figures S6 and S7.