Abstract
Background
Surgery followed by chemotherapy is the primary modality of cure for patients with resectable pancreatic cancer but is associated with significant morbidity. The aim of the present study was to evaluate the role of cardiopulmonary exercise testing (CPET) in predicting post-operative adverse events and fitness for chemotherapy after major pancreatic surgery.
Methods
Patients who underwent a pancreaticoduodenectomy or total pancreatectomy for pancreatic head lesions and had undergone pre-operative CPET were included in this retrospective study. Data on patient demographics, comorbidity and results of pre-operative evaluation were collected. Post-operative adverse events, hospital stay and receipt of adjuvant therapy were outcome measures.
Results
One hundred patients were included. Patients with an anaerobic threshold less than 10 ml/kg/min had a significantly greater incidence of a post-operative pancreatic fistula [International Study Group for Pancreatic Surgery (ISGPS) Grades A–C, 35.4% versus 16%, P = 0.028] and major intra-abdominal abscesses [Clavien–Dindo (CD) Grades III–V, 22.4% versus 7.8%, P = 0.042] and were less likely to receive adjuvant therapy [hazard ratio (HR) 6.30, 95% confidence interval (CI) 1.25–31.75, P = 0.026]. A low anaerobic threshold was also associated with a prolonged hospital stay (median 20 versus 14 days, P = 0.005) but not with other adverse events.
Discussion
CPET predicts a post-operative pancreatic fistula, major intra-abdominal abscesses as well as length of hospital stay after major pancreatic surgery. Patients with a low anaerobic threshold are less likely to receive adjuvant therapy.
Introduction
Pancreatic cancer is the 10th most common cancer in the UK but the fifth most common cause of cancer death with only 16–17% surviving beyond the first year and 3% surviving beyond 5 years.1 The majority of patients (80–85%) with pancreatic cancer present with inoperable disease.1,2 In patients with resectable disease, surgery2–4 followed by adjuvant chemotherapy5,6 remains the primary modality of cure.
The decision to operate on these patients depends not only on the pre-operative tumour stage but also on patient factors.7,8 Patient factors, in particular those that affect fitness, are also important in determining the short-term outcome in those that do undergo potentially curative surgery.9,10 However, major pancreatic surgery is associated with significant morbidity and mortality and patients who have post-operative complications are less likely to get adjuvant therapy.11
There have been a number of attempts to objectively define patient fitness and its relationship with post-operative outcome. Copeland et al. (1991) reported that the Physiological and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM) criteria, in particular the POSSUM physiology score (PPS) could be used to quantify the risk of post-operative morbidity and mortality.12 However, the role of POSSUM in predicting post-operative outcome after surgery for pancreatic cancer is not entirely clear.13–17 The physiological component of POSSUM as well as other similar risk scoring systems such as E-PASS (Estimation of Physiologic Ability and Surgical Stress)18 are calculated based on known comorbidities, clinically evident abnormalities in patient physiology or blood tests.
More recently, there has been some evidence that the presence of an ongoing systemic inflammatory response before surgery is associated with the development of post-operative complications in patients undergoing surgery for colorectal cancer,19 oesophageal cancer20 as well as pancreatic cancer.21
Older et al. (1993) reported that cardiopulmonary exercise testing (CPET) was an objective evaluation of the response of the cardiovascular and respiratory systems to an increase in oxygen demand during exercise and was useful in predicting peri-operative morbidity and mortality in patients undergoing major abdominal surgery.22
The aim of the present study was to evaluate the role of various measures of patient physiological fitness including cardiopulmonary exercise testing in predicting post-operative adverse events as well as fitness for adjuvant therapy in patients undergoing major pancreatic surgery.
Methods
Patients who underwent a pancreaticoduodenectomy or total pancreatectomy for pancreatic head lesions between August 2008, when cardiopulmonary exercise testing was first used for fitness assessment at our hospital, and January 2012 were considered for this retrospective study. Patients who had not undergone cardiopulmonary exercise testing as part of their preoperative assessment and patients who underwent cardiopulmonary exercise testing but did not undergo surgery were excluded.
Data on patient demographics, comorbidity including cardiovascular and respiratory disease, pre-operative blood tests, chest X-ray and cardiopulmonary exercise tests were collected from prospectively maintained databases (March 2009–January 2012) and case note review (August 2008–March 2009). Data were also collected for patients who did not undergo cardiopulmonary exercise testing to allow comparison with the study group. The POSSUM Physiology Score was calculated based on 11 physiological parameters [cardiac disease including hypertension, ischaemic heart disease and heart failure, respiratory disease causing breathlessness on exertion and chronic obstructive pulmonary disease (COPD), electrocardiography (ECG) changes, pulse rate, blood pressure, haemoglobin, white cell count, serum sodium, serum potassium, serum urea and Glasgow Coma Scale] as described previously.
Cardiopulmonary exercise tests were performed in the Department of Respiratory Medicine at the Glasgow Royal Infirmary using the ZAN-600 CPET suite (nSpire Health, Longmont, CO, USA). An electrically-braked cycle ergometer was used to perform a symptom-limited, incremental work-load test preceded by a 3-min rest period. The test was stopped at maximum exercise tolerance, significant ischaemic changes on ECG or for other safety reasons. The anaerobic threshold was calculated using the V-slope23,24 and ventilatory equivalents23 methods. A low anaerobic threshold was defined as oxygen consumption less than 10 ml/kg/min based on previous work by Snowden et al.25 who reported that an anaerobic threshold less than 10.1 ml/kg/min was associated with an increase in post-operative complications after major abdominal surgery.
The decision to operate was based on overall pre-operative evaluation of the patient's comorbid conditions and performance status and not exclusively on the result of cardiopulmonary exercise testing. While the results of cardiopulmonary exercise tests were available to the clinicians before surgery, no specific changes were made to peri-operative management based exclusively on these results. These results were used in conjunction with other established forms of pre-operative evaluation for risk assessment and peri-operative care. All patients were routinely admitted to the surgical high-dependency unit unless intra-operative events or post-operative complications required admission to the intensive care unit. Patients were discharged after resolution of organ dysfunction and/or sepsis and when nutrition, analgesia and mobilization were adequately established to the clinician's and patient's satisfaction.
Post-operative adverse events were recorded using internationally recognized definitions. The International Study Group for Pancreatic Surgery (ISGPS) definitions were used to classify pancreatic fistulae26 and post-operative haemorrhage.27 The Clavien–Dindo (CD) classification28,29 was used to grade other complications and CD grades III–V were considered major. Multiple admissions to critical care as well as re-operations were recorded. Operative mortality was defined as post-operative death in-hospital regardless of duration of stay or occurring within 30 days of the surgery. All complications were discussed at a weekly multidisciplinary meeting attended by three pancreatic surgeons and a radiologist with a specialist interest in pancreatic diseases and recorded in a prospective database.
Primary outcome measures were length of stay in hospital, major post-operative adverse events including operative mortality and fitness to undergo adjuvant therapy when indicated. Secondary outcome measures included cumulative length of stay in critical care and the number of critical care admissions.
Statistical analysis
Grouping of the variables was carried out using standard or previously published thresholds. In the absence of such thresholds, the variables were treated as continuous variables and analysed using non-parametric statistical methods. Cox proportional hazards regression analysis was used to study the relationship between pre-operative risk factors and length of hospital stay. The chi-square test was used to examine the relationship between complications and anaerobic threshold as a categorical variable. Univariate binary logistic regression analysis with a calculation of hazard ratios (HR) and 95% confidence intervals (CIs) was used to explore the association between peri-operative clinico-pathological factors and receipt of adjuvant therapy. Multivariate binary logistic regression analysis was performed on all variables showing a significant association on univariate analysis. Backward stepwise regression was used starting with a saturated model and variables with P-value > 0.1 were excluded at each step until no more variables could be excluded. SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis.
Results
One hundred and twenty-nine patients had undergone a pancreaticoduodenectomy (n = 127), sub-total pancreatectomy (n = 1) or total pancreatectomy (n = 1) during the study period. Sub-total and total pancreatectomies were performed in patients scheduled for a pancreaticoduodenectomy but were found to have pancreatic remnants either too friable or too atrophic during the operation to perform an anastomosis. Of these, 100 patients (pancreaticoduodenectomy, 98 and sub-total/total pancreatectomy, 2) had undergone cardiopulmonary exercise testing as part of their pre-operative assessment and were included in the study. Pathological examination of the resected specimen showed pancreatic ductal adenocarcinoma (n = 37), ampullary adenocarcinoma (n = 18), cholangiocarcinoma (n = 17), duodenal adenocarcinoma (n = 6), intraductal papillary mucinous neoplasia (n = 4), neuroendocrine tumours (n = 7), other neoplasia (n = 4) or chronic pancreatitis (n = 2).
Twenty-nine patients did not undergo cardiopulmonary exercise testing owing to reasons including subjective assessment of fitness, resource constraints and logistics. Table 1 shows the clinico-pathological characteristics of patients included in the study compared with the excluded patients. The median age in the study cohort was higher than in the excluded cohort (66 versus 54 years, P = 0.001). However, there was no difference in gender, body mass index, pre-operative biliary drainage, jaundice at the time of surgery, modified Glasgow Prognostic Score, POSSUM physiology score, pre-operative blood tests including haemoglobin and liver function tests and length of critical care/hospital stay. The overall post-operative mortality during the study period was 5.4% (7/129) with all deaths occurring in the study cohort (P = 0.144).
Table 1.
Clinico-pathological characteristics of patients undergoing major pancreatic surgery during the study period
| All patients | Excluded | Included | P | |
|---|---|---|---|---|
| n = 129 | n = 29 | n = 100 | ||
| Age (years) | ||||
| ≤65 | 71 (55%) | 24 | 47 | 0.001 |
| >65 | 58 (45%) | 5 | 53 | |
| Gender | ||||
| Male | 77 (60%) | 17 | 60 | 0.894 |
| Female | 52 (40%) | 12 | 40 | |
| BMI (kg/m2) | ||||
| ≤25 | 53 (44%) | 8 | 45 | 0.817 |
| >25 | 66 (56%) | 11 | 55 | |
| Pre-operative biliary drainage | ||||
| No | 68 (59%) | 12 | 56 | 0.154 |
| Yes | 48 (41%) | 4 | 44 | |
| mGPS | ||||
| 0 | 76 (59%) | 13 | 63 | 0.279 |
| 1 | 11 (9%) | 5 | 6 | |
| 2 | 41 (32.0%) | 10 | 31 | |
| Haemoglobin (g/dl) | ||||
| ≥12 | 80 (64%) | 18 | 62 | 0.353 |
| <12 | 45 (36%) | 7 | 38 | |
| POSSUM Physiology Score | ||||
| 11–14 | 61 (51%) | 12 | 50 | 0.701 |
| >14 | 59 (49%) | 10 | 50 | |
| Serum bilirubin (micromol/l) | ||||
| ≤35 | 70 (55%) | 12 | 58 | 0.156 |
| >35 | 58 (45%) | 16 | 42 | |
| Operation type | ||||
| Pancreatico-duodenectomy | 127 (98%) | 29 | 98 | 0.045 |
| (Sub-)Total Pancreatectomy | 2 (2%) | 0 | 2 | |
| Operative mortality | 7 (5%) | 0 | 7 | 0.144 |
| Postoperative stay (days) | 17 (13–27) | 20 (13–30) | 17 (13–26) | 0.518 |
| Critical care stay (days) | 7 (6–12) | 7 (6–14) | 7 (6–12) | 0.448 |
Values are either median (inter-quartile range) with P statistic using the Mann–Whitney test or number of patients (percentage) with P statistic using the chi-square test.
The median anaerobic threshold was 10.3 ml/kg/min (inter-quartile range, IQR 8.8–11.6). The anaerobic threshold was less than 10 ml/kg/min in 49 patients. The distribution of anaerobic threshold across the study cohort is shown in Fig. 1.
Figure 1.
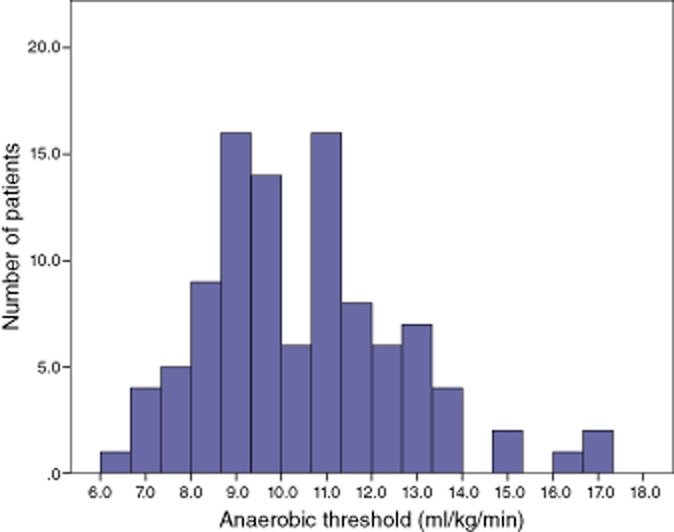
Distribution of anaerobic threshold across the study population
The relationship between anaerobic threshold and major post-operative adverse events including mortality is shown in Table 2. Patients with an anaerobic threshold less than 10 ml/kg/min had a significantly greater incidence of post-operative pancreatic fistula (35.4% versus 16%, P = 0.028) as well as major intra-abdominal abscesses (CD Grade III–V, 22.4% versus 7.8%, P = 0.042). While there was an association between low anaerobic threshold and grade of pancreatic fistula, this was not statistically significant (P = 0.091).
Table 2.
The relationship between the anaerobic threshold and complications in patients undergoing major pancreatic surgery
| Anaerobic threshold (ml/kg/min) | |||||
|---|---|---|---|---|---|
| Complications | ≥10 | <10 | P | ||
| n | n | ||||
| Cardiac complications | |||||
| Grade 0–II | 99 | 51 | 48 | 0.308 | |
| Grade III–V | 1 | 0 | 1 | ||
| Respiratory complications | |||||
| Grade 0–II | 93 | 48 | 45 | 0.657 | |
| Grade III–V | 7 | 3 | 4 | ||
| Intra-abdominal abscess | |||||
| Grade 0–II | 85 | 47 | 38 | 0.042 | |
| Grade III–V | 15 | 4 | 11 | ||
| Pancreatic fistula (total/sub-total pancreatectomies excluded) | |||||
| No | 73 | 42 | 31 | 0.028 | |
| Yes | 25 | 8 | 17 | ||
| Pancreatic fistula (ISGPS Classification) | |||||
| No | 73 | 42 | 31 | 0.091 | |
| Grade A | 9 | 3 | 6 | ||
| Grade B | 8 | 1 | 7 | ||
| Grade C | 8 | 4 | 4 | ||
| Post-pancreatectomy haemorrhage (ISGPS Classification) | |||||
| No | 84 | 41 | 43 | 0.207 | |
| Grade A | 4 | 2 | 2 | ||
| Grade B | 4 | 2 | 2 | ||
| Grade C | 8 | 6 | 2 | ||
| Admissions to critical care | |||||
| 1 | 74 | 38 | 36 | 0.906 | |
| >1 | 26 | 13 | 13 | ||
| Re-operation | |||||
| No | 89 | 47 | 42 | 0.306 | |
| Yes | 11 | 4 | 7 | ||
| Operative mortality | |||||
| No | 93 | 47 | 46 | 0.737 | |
| Yes | 7 | 4 | 3 | ||
There was no association between low anaerobic threshold and cardiopulmonary complications or post-operative mortality. Major cardiopulmonary complications occurred more often in patients with major intra-abdominal adverse events including major intra-abdominal abscesses or Grade B and C pancreatic fistulae or haemorrhage than in patients who did not have these complications (5/31, 16.1% versus 2/69, 2.9%, P = 0.017). Post-operative mortality was not associated with anaerobic threshold (HR 0.77, 95% CI 0.16–3.61, p 0.737) or the POSSUM Physiology Score (HR 0.39, 95% CI 0.07–2.12, P = 0.277). Post-operative mortality was associated with post-operative pancreatic fistula (n = 5), post-pancreatectomy haemorrhage (n = 3), major intra-abdominal sepsis (n = 6) and major cardiorespiratory complications (n = 4) with six patients requiring radiological or operative intervention.
The median length of post-operative stay was 17 days (IQR 13–26). The median cumulative length of stay in critical care was 7 days (IQR 6–12). Twenty-six patients were admitted to critical care more than once. The relationship between pre-operative clinico-pathological characteristics and length of post-operative stay in patients who were discharged from hospital (n = 93) is shown in Table 3. On univariate analysis, age over 65 years (P = 0.072) and low anaerobic threshold (P = 0.010) were associated with prolonged post-operative stay. On multivariate Cox proportional hazards regression analysis, an anaerobic threshold less than 10 ml/kg/min (hazard ratio 1.74, 95% CIs 1.14–2.65, P = 0.010) was the only significant factor associated with prolonged post-operative stay. A Kaplan–Meier plot for the probability of remaining in hospital over time for patients with low and normal anaerobic thresholds is shown in Fig. 2. Patients with a low anaerobic threshold stayed a median 6 days longer in hospital (14 versus 20 days, Mann–Whitney U-test P = 0.001). There was no significant association between any of the pre-operative factors including anaerobic threshold and length of critical care stay or number of critical care admissions.
Table 3.
The relationship between clinico-pathological characteristics and post-operative stay in patients (excluding operative mortality) undergoing major pancreatic surgery (n = 93): Cox's regression analysis
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||||
| ≤65 | 44 | |||||||
| >65 | 49 | 1.47 | 0.97–2.24 | 0.072 | 1.48 | 0.97–2.25 | 0.068 | |
| Gender | ||||||||
| Male | 56 | |||||||
| Female | 37 | 1.32 | 0.86–2.03 | 0.199 | ||||
| BMI (kg/m2) | ||||||||
| ≤25 | 42 | |||||||
| >25 | 51 | 0.87 | 0.58–1.32 | 0.512 | ||||
| Smoking | ||||||||
| No | 56 | |||||||
| Yes | 37 | 1.26 | 0.82–1.94 | 0.294 | ||||
| POSSUM Physiology Score | ||||||||
| ≤14 | 45 | |||||||
| >14 | 48 | 1.28 | 0.85–1.95 | 0.240 | ||||
| Pre-operative biliary drainage | ||||||||
| No | 53 | |||||||
| Yes | 40 | 1.08 | 0.71–1.65 | 0.724 | ||||
| Serum bilirubin (micromol/l) | ||||||||
| ≤35 | 54 | |||||||
| >35 | 39 | 1.26 | 0.83–1.92 | 0.277 | ||||
| mGPS | ||||||||
| 0 | 59 | |||||||
| 1 | 5 | 1.22 | 0.78–1.92 | 0.387 | ||||
| 2 | 29 | 1.87 | 0.71–4.88 | 0.204 | ||||
| Haemoglobin (g/dl) | ||||||||
| ≥12 | 57 | |||||||
| <12 | 36 | 1.19 | 0.78–1.81 | 0.422 | ||||
| Anaerobic threshold (ml/kg/min) | ||||||||
| ≥10 | 47 | |||||||
| <10 | 46 | 1.74 | 1.14–2.64 | 0.010 | 1.74 | 1.14–2.65 | 0.010 | |
| Anaerobic threshold (ml/kg/min) | ||||||||
| ≥11 | 33 | |||||||
| <11 | 60 | 1.44 | 0.94–2.22 | 0.097 | 0.395 | |||
Figure 2.
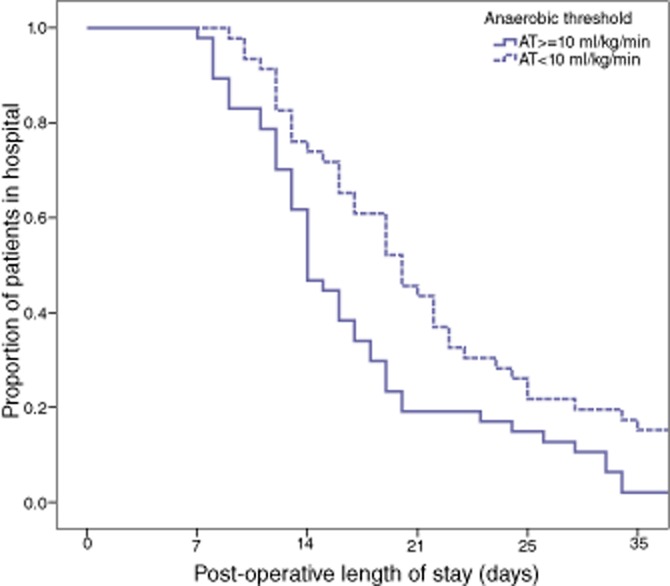
Kaplan–Meier Plot of post-operative length of stay in patients with an anaerobic threshold ≥10 ml/kg/min compared with <10 ml/kg/min
The relationship between clinico-pathological patient factors and receipt of adjuvant therapy is shown in Table 4. Fifty-five patients were included in the analysis. Patients were excluded if chemotherapy was not indicated (n = 28), in the event of operative mortality (n = 7), if chemotherapy was offered but declined by the patient (n = 4), or where they had not been seen by an oncologist yet (n = 6). On binary logistic regression analysis, an anaerobic threshold less than 10 ml/kg/min was the only pre-operative factor that was associated with non-receipt of adjuvant therapy (HR 6.30, 95% CI 1.25–31.75, P = 0.026).
Table 4.
The relationship between clinico-pathological characteristics and receipt of adjuvant therapy in patients undergoing major pancreatic surgery (n = 55): binary logistic regression
| Univariate | |||||
|---|---|---|---|---|---|
| Variable | n = 55 | HR | 95% CI | P | |
| Age (years) | |||||
| ≤65 | 25 | ||||
| >65 | 30 | 2.63 | 0.71–9.74 | 0.149 | |
| Gender | |||||
| Male | 31 | ||||
| Female | 24 | 2.08 | 0.61–7.13 | 0.242 | |
| BMI (kg/m2) | |||||
| ≤25 | 25 | ||||
| >25 | 30 | 0.78 | 0.23–2.64 | 0.693 | |
| Smoking | |||||
| No | 35 | ||||
| Yes | 20 | 0.96 | 0.27–3.41 | 0.953 | |
| POSSUM physiology score | |||||
| ≤14 | 25 | ||||
| >14 | 30 | 1.63 | 0.46–5.73 | 0.447 | |
| Pre-operative biliary drainage | |||||
| No | 27 | ||||
| Yes | 28 | 0.95 | 0.28–3.21 | 0.937 | |
| Serum bilirubin (micromol/l) | |||||
| ≤35 | 27 | ||||
| >35 | 28 | 2.08 | 0.60–7.30 | 0.251 | |
| mGPS | |||||
| 0 | 32 | ||||
| 1 | 2 | 0.0 | 0.0 | ||
| 2 | 21 | 1.20 | 0.35–4.15 | 0.773 | |
| Haemoglobin (g/dl) | |||||
| ≥12 | 31 | ||||
| <12 | 24 | 0.96 | 0.28–3.26 | 0.946 | |
| Anaerobic threshold (ml/kg/min) | |||||
| ≥10 | 23 | ||||
| <10 | 32 | 6.30 | 1.25–31.75 | 0.026 | |
| Anaerobic threshold (ml/kg/min) | |||||
| ≥11 | 16 | ||||
| <11 | 39 | 3.11 | 0.61–15.88 | 0.172 | |
Discussion
The results of the present study show that a low anaerobic threshold is associated with prolonged post-operative stay in hospital, post-operative pancreatic fistula and intra-abdominal abscesses in patients undergoing major resections for pancreatic head lesions. The results of this study also show that patients with a low anaerobic threshold are less likely to receive adjuvant therapy.
Therefore, it would appear that objective measurement of patient physiological fitness using cardiopulmonary exercise testing is superior to conventional measures of patient fitness including the POSSUM Physiology Score or the modified Glasgow Prognostic Score and may have a role in predicting short-term outcome which in turn affects the overall management of these patients including receipt of adjuvant therapy.
Patients with a low anaerobic threshold stayed longer in hospital after their operation. While length of stay in hospital is influenced by multiple factors including post-operative complications, it would appear that patients with a low anaerobic threshold take longer to recover from the physiological stress placed by major pancreatic surgery and its sequelae.
The incidence of a pancreatic fistula was greater in patients with a low anaerobic threshold. This association needs further evaluation taking into consideration other well-recognised risk factors for pancreatic fistula such as pancreatic texture, pancreatic duct size and intra-operative blood loss.30–32 It is possible that local or operative factors may be compounded by poor oxygen delivery and organ perfusion as measured by cardiopulmonary exercise testing. There was a non-significant trend towards clinically relevant pancreatic fistulae (ISGPS Grades B and C) as well as a significant association with major intra-abdominal abscesses (CD Grades 3–5, i.e. requiring intervention, associated with organ dysfunction requiring intensive care or resulting in mortality). This would suggest that complications in patients with a low anaerobic threshold are more likely to be severe than in patients with normal anaerobic threshold. However, there was no difference in mortality between patients with a normal or low anaerobic threshold, indicating that multiple factors including pre-operative patient fitness, local and operative factors, systemic inflammatory response, number of complications as well as peri-operative critical care all play a role.
The results of this study also show that patients with a low anaerobic threshold were less likely to receive adjuvant therapy. Adjuvant therapy in patients undergoing pancreatic resections for cancer has been shown in multiple randomized trials to improve survival significantly.5,6 While post-operative mortality after pancreatic surgery has steadily improved over the years with major improvements in the quality of surgical and critical care over the past decade31 even in elderly patients,33 post-operative morbidity remains high.10 The results of this study show that poor pre-operative fitnees is not only associated with a protracted protracted post-operative course with complications but also with non-receipt of adjuvant therapy.
In the present study, the anaerobic threshold was less than 10 ml/kg/min in 49% of patients and less than 11 ml/kg/min in 64% of patients. The proportion of patients with an anaerobic threshold less than 11 ml/kg/min in this study was much greater than reported in studies involving patients undergoing oesophageal surgery (16%),34 liver transplantation (39%)35 or other major abdominal surgery (29%)22 and may indicate the poor pre-operative fitness levels of patients undergoing major pancreatic surgery at our unit. While several previous studies have shown that low anaerobic threshold and/or low VO2 peak are associated with post-operative complications or prolonged hospital stay after major abdominal surgery as well as non-abdominal surgery,22,35–39 others have disputed this.34,40,41 Older et al. reported in 1993 that a low anaerobic threshold less than 11 ml/kg/min was associated with a significantly higher risk of postoperative mortality from cardiovascular causes in a series of 187 elderly patients undergoing major abdominal surgery.22 However, Snowden et al.25 reported that patients with an anaerobic threshold less than 10.1 ml/kg/min had significantly greater cardiopulmonary complications as well as non-cardiopulmonary and infectious complications whereas Forshaw et al.34 reported that using a cut-off of 11 ml/kg/min for the anaerobic threshold did not predict post-operative adverse events less after an oesophagectomy. The lack of an association between a low anaerobic threshold and cardiopulmonary complications in this study may have been due to two reasons. Major cardiopulmonary complications occurred more often in association with major intra-abdominal adverse events which are determined largely by pancreatic morphology and local anatomy.30 Moreover, the stringent fitness criteria for undergoing a pancreaticoduodenectomy may have excluded patients with known co-morbid cardiorespiratory diseases such as severe chronic obstructive pulmonary disease or cardiac failure.
The results of this study are consistent with the results of the previous study by Ausania et al.42 who reported an increased incidence of pancreatic fistula and prolonged post-operative stay in patients with an anaerobic threshold less than 10.1 ml/kg/min. However, this study did not report the association between anaerobic threshold and receipt of adjuvant therapy.
The physiological demands placed on a patient undergoing major pancreatic surgery are significant, both during and after the operation. It is not entirely surprising therefore, that conventional parameters of patient fitness such as the POSSUM Physiology Score or the modified Glasgow Prognostic Score are limited in their ability to distinguish patients based on their performance under physiological stress. Cardiopulmonary exercise testing overcomes this disadvantage by replicating some of the physiological burden major pancreatic surgery places on the functional capacity of the patient's cardiovascular and respiratory systems.
This functional capacity of patients to withstand the physiological burden of major surgery can be improved by the process of ‘prehabilitation’.43 It has been suggested that prehabilitation not only improves aerobic capacity44 but may also improve post-operative recovery.45,46 The results of this study show that impaired aerobic capacity is associated with post-operative adverse events. Therefore, it would appear that prehabilitation using interventions such as exercise and nutrition, by improving physiological fitness, may have a role in improving post-operative outcomes after major pancreatic surgery and may improve the proportion of patients receiving adjuvant therapy.
Further work needs to be carried out to study the value of cardiopulmonary exercise testing in predicting post-operative complications in conjunction with previously established factors such as pancreatic morphology and operative factors before it can be used on its own to select or exclude patients for a pancreaticoduodenectomy. Cardiopulmonary exercise testing would play an important role not only in identifying patients who will benefit from prehabilitation, but also in the objective measurement of the effects of such interventions on aerobic capacity as well as in identifying high-risk patients who may not be able to complete oncological treatment. Prehabilitation and optimized peri-operative care may allow a greater proportion of high-risk patients to progress to oncological treatment after surgery.
Acknowledgments
The authors wish to acknowledge the role of Dr J. Edwards and Dr C. Pow of the Section of Anaesthesia, Glasgow Royal Infirmary in helping with the conduct of the very first cardiopulmonary exercise tests at the Glasgow Royal Infirmary as well as Dr Janan Noman of the Department of Respiratory Medicine, Glasgow Royal Infirmary who helped with ongoing cardiopulmonary exercise testing.
Conflicts of interest
None declared.
References
- 1. Cancer research uk . Available at http://info.cancerresearchuk.org/cancerstats/types/pancreas/ (last accessed January 2012)
- 2.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the national cancer database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 4.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. ; discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the espac-1 and -3(v1) trials. Br J Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 7.Sandroussi C, Brace C, Kennedy ED, Baxter NN, Gallinger S, Wei AC. Sociodemographics and comorbidities influence decisions to undergo pancreatic resection for neoplastic lesions. J Gastrointest Surg. 2010;14:1401–1408. doi: 10.1007/s11605-010-1255-2. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann CD, Palser T, Briggs CD, Cameron I, Rees M, Buckles J, et al. A review of factors predicting perioperative death and early outcome in hepatopancreaticobiliary cancer surgery. HPB. 2010;12:380–388. doi: 10.1111/j.1477-2574.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teh SH, Diggs BS, Deveney CW, Sheppard BC. Patient and hospital characteristics on the variance of perioperative outcomes for pancreatic resection in the united states: a plea for outcome-based and not volume-based referral guidelines. Arch Surg. 2009;144:713–721. doi: 10.1001/archsurg.2009.67. [DOI] [PubMed] [Google Scholar]
- 12.Copeland GP, Jones D, Walters M. Possum: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 13.de Castro SMM, Houwert JT, Lagarde SM, Reitsma JB, Busch ORC. Gulik TM, et al. Evaluation of possum for patients undergoing pancreatoduodenectomy. World J Surg. 2009;33:1481–1487. doi: 10.1007/s00268-009-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamijmarane A, Bhati CS, Mirza DF, Bramhall SR, Mayer DA, Wigmore SJ, et al. Application of portsmouth modification of physiological and operative severity scoring system for enumeration of morbidity and mortality (p-possum) in pancreatic surgery. World J Surg Oncol. 2008;6:39. doi: 10.1186/1477-7819-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratt W, Joseph S, Callery MP, Vollmer CMJ. Possum accurately predicts morbidity for pancreatic resection. Surgery. 2008;143:8–19. doi: 10.1016/j.surg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Kocher HM, Tekkis PP, Gopal P, Patel AG, Cottam S, Benjamin IS. Risk-adjustment in hepatobiliary pancreatic surgery. World J Gastroenterol. 2005;11:2450–2455. doi: 10.3748/wjg.v11.i16.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AW, Shah SR, Agarwal AK, Davidson BR. Evaluation of the possum scoring system for comparative audit in pancreatic surgery. Dig Surg. 2003;20:539–545. doi: 10.1159/000073701. [DOI] [PubMed] [Google Scholar]
- 18.Haga Y, Ikei S, Ogawa M. Estimation of physiologic ability and surgical stress (e-pass) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–225. doi: 10.1007/BF02483010. [DOI] [PubMed] [Google Scholar]
- 19.Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100:1236–1239. doi: 10.1038/sj.bjc.6604997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, et al. Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2010;18:1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 21.Knight BC, Kausar A, Manu M, Ammori BA, Sherlock DJ, O'Reilly DA. Evaluation of surgical outcome scores according to isgps definitions in patients undergoing pancreatic resection. Dig Surg. 2010;27:367–374. doi: 10.1159/000313693. [DOI] [PubMed] [Google Scholar]
- 22.Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993;104:701–704. doi: 10.1378/chest.104.3.701. [DOI] [PubMed] [Google Scholar]
- 23.Sue DY, Wasserman K, Moricca RB, Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. use of the v-slope method for anaerobic threshold determination. Chest. 1988;94:931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- 24.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 25.Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535–541. doi: 10.1097/SLA.0b013e3181cf811d. [DOI] [PubMed] [Google Scholar]
- 26.2005. Postoperative pancreatic fistula: an international study group (isgpf) definition.
- 27.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (pph): an international study group of pancreatic surgery (isgps) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clavien PA, Barkun J. Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 30.Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–707. doi: 10.1097/SLA.0b013e31823598fb. ; discussion 707–8. [DOI] [PubMed] [Google Scholar]
- 31.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. ; discussion 1210–1. [DOI] [PubMed] [Google Scholar]
- 32.Pratt WB, Callery MP, Vollmer CMJ. Risk prediction for development of pancreatic fistula using the isgpf classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 33.Makary MA, Winter JM, Cameron JL, Campbell KA, Chang D, Cunningham SC, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Forshaw MJ, Strauss DC, Davies AR, Wilson D, Lams B, Pearce A, et al. Is cardiopulmonary exercise testing a useful test before esophagectomy? Ann Thorac Surg. 2008;85:294–299. doi: 10.1016/j.athoracsur.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 35.Epstein SK, Freeman RB, Khayat A, Unterborn JN, Pratt DS, Kaplan MM. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl. 2004;10:418–424. doi: 10.1002/lt.20088. [DOI] [PubMed] [Google Scholar]
- 36.McCullough PA, Gallagher MJ, Dejong AT, Sandberg KR, Trivax JE, Alexander D, et al. Cardiorespiratory fitness and short-term complications after bariatric surgery. Chest. 2006;130:517–525. doi: 10.1378/chest.130.2.517. [DOI] [PubMed] [Google Scholar]
- 37.Nagamatsu Y, Shima I, Yamana H, Fujita H, Shirouzu K, Ishitake T. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg. 2001;121:1064–1068. doi: 10.1067/mtc.2001.113596. [DOI] [PubMed] [Google Scholar]
- 38.Older P, Hall A. Clinical review: how to identify high-risk surgical patients. Crit Care. 2004;8:369–372. doi: 10.1186/cc2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 40.Hightower CE, Riedel BJ, Feig BW, Morris GS, Ensor JEJ, Woodruff VD, et al. A pilot study evaluating predictors of postoperative outcomes after major abdominal surgery: physiological capacity compared with the asa physical status classification system. Br J Anaesth. 2010;104:465–471. doi: 10.1093/bja/aeq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton RA, Bannard-Smith JP, Washington SJ, Wisely N, Columb M, Rees L. Cardiopulmonary exercise testing and length of stay in patients undergoing major surgery. Anaesthesia. 2011;66:393–394. doi: 10.1111/j.1365-2044.2011.06725.x. [DOI] [PubMed] [Google Scholar]
- 42.Ausania F, Snowden CP, Prentis JM, Holmes LR, Jaques BC, White SA, et al. Effects of low cardiopulmonary reserve on pancreatic leak following pancreaticoduodenectomy. Br J Surg. 2012;99:1290–1294. doi: 10.1002/bjs.8859. [DOI] [PubMed] [Google Scholar]
- 43.Topp R, Ditmyer M, King K, Doherty K. Hornyak J3. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13:263–276. doi: 10.1097/00044067-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 45.Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg. 2011;17:461–468. doi: 10.5761/atcs.oa.11.01663. [DOI] [PubMed] [Google Scholar]
- 46.Mayo NE, Feldman L, Scott S, Zavorsky G, Kim DJ, Charlebois P, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]


