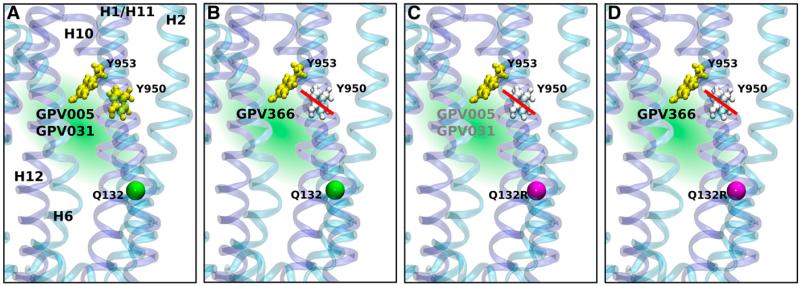
Fig. 5.
Schematic presentation of the importance of tyrosine hydroxyl groups (tyrosines, yellow) for interaction with propafenone analogs in the outward-facing P-gp structure (template Sav1866) (A and B); Cα atom of residue Q132 (shown as a green sphere) and after introduction of the R132 selector mutation (Cα atom of residue Q132 shown in magenta) (C and D). The hydroxyl groups of both Y950 and Y953 are able to form hydrogen bonds with protonatable propafenones GPV005 and GPV031 (A), whereas compound GPV366 only forms hydrogen bonds with residue Y953 (B). The gray color of residue Y950 and red bar indicate that hydrogen bonds of residue Y950 are not important for interaction. The presence of the selector residue R132 (C and D) eliminates the importance of the hydroxyl group of Y950 for propafenone analog binding, whereas the interaction of residue Y953 continues to contribute to the interaction. Use of gray type indicates incomplete prevention of access of compounds GPV005 and GPV031 in C.