Abstract
ClC-7 is a 2Cl−/1H+-exchanger expressed at late endosomes and lysosomes, as well as the ruffled border of osteoclasts. ClC-7 deficiencies in mice and humans lead to impaired osteoclast function and therefore osteopetrosis. Failure of tooth eruption is also apparent in ClC-7 mutant animals, and this has been attributed to the osteoclast dysfunction and the subsequent defect in alveolar bone resorptive activity surrounding tooth roots. Ameloblasts also express ClC-7, and this study aims to determine the significance of ClC-7 in enamel formation by examining the dentitions of ClC-7 mutant mice. Micro-CT analysis revealed that the molar teeth of 3-week old ClC-7 mutant mice had no roots, and the incisors were smaller than their age-matched controls. Despite these notable developmental differences, the enamel and dentin densities of the mutant mice were comparable to those of the wild type littermates. Scanning electron microscopy (SEM) showed normal enamel crystallite and prismatic organization in the ClC-7 mutant mice, although the enamel was thinner (hypoplastic) than in controls. These results suggested that ClC-7 was not critical to enamel and dentin formation, and the observed tooth defects may be related more to a resulting alveolar bone phenotype. Micro-CT analysis also revealed abnormal features in the calvarial bones of the mutant mice. The cranial sutures in ClC-7 mutant mice remained open compared to the closed sutures seen in the control mice at 3 weeks. These data demonstrate that ClC-7 deficiency impacts the development of the dentition and calvaria, but does not significantly disrupt amelogenesis.
Keywords: ameloblast, amelogenesis, biomineralization, craniofacial development, pH regulation, enamel, chloride channels
INTRODUCTION
ClC-7, encoded by Clcn7 gene, is a voltage-gated 2Cl−/1H+-exchanger (Leisle et al., 2011), and appears to be ubiquitously expressed in all cells examined (Brandt and Jentsch, 1995). The subcellular localization of ClC-7 is at late endosomes and lysosomes, as well as the ruffled border of osteoclasts (Kornak et al., 2001; Kasper et al., 2005). Osteoclasts degrade and resorb bone matrix using a specialized cell compartment, the resorptive lacuna, in which an acidic pH is established by V-type ATPase (V-ATPase) present in the ruffled membrane (Marshansky et al., 2014). The H+ transport by V-ATPase is electrogenic and requires an anion channel in the same membrane to balance the charges of ion across the membrane (Jentsch, 2007; Stauber and Jentsch, 2013). Loss of Clcn7 in mice (Clcn7−/−) causes lysosomal storage disease (Kasper et al., 2005; Wartosch et al., 2009) and osteopetrosis as osteoclasts fail to form a ruffled border and properly acidify the extracellular space between the osteoclast and the bone (Kornak et al., 2001; Neutzsky-Wulff et al., 2010). Similarly, mutations in the CLCN7 gene in humans led to autosomal recessive osteopetrosis (ARO), also known as malignant infantile osteopetrosis (Cleiren et al., 2001; Kornak et al., 2001). Certain missense mutations in one allele of CLCN7 gene lead to autosomal dominant osteopetrosis type 2 (ADO2, Albers-Schonberg disease), a milder and the most common form of osteopetrosis with symptoms not evident until later in life (Cleiren et al., 2001; Del Fattore et al., 2008). Not surprisingly, lack of tooth eruption has been reported in both Clcn7−/− mice (Kornak et al., 2001) and in patients with CLCN7 gene mutations (Xue et al., 2012; Duan, 2014); this is because tooth eruption is critically dependent on osteoclast activity to reabsorb surrounding alveolar bone, which enables root development and the crown of the tooth to erupt into the oral cavity.
In this study we examined whether ClC-7 is also directly involved in amelogenesis, based on the fact that ameloblasts, like osteoclasts, express V-ATPase (Lin et al., 1994; Josephsen et al., 2010; Damkier et al., 2014) and ClC-7 (Lacruz et al., 2013). In addition, the acidic pH of the enamel matrix bordering ruffle-ended ameloblasts (RA) has long been observed (Smith, 1998), although it remains unclear whether the acidic pH is the result of active acid secretion by ameloblasts, or is the by-product of hydroxyapatite crystal synthesis (Damkier et al., 2014). Although a clear correlation exits between osteopetrosis phenotype and the delay or absence of tooth eruption, the structure of the tooth and surrounding bone in osteopetrotic animal models has never been examined in detail. In this study, micro-CT (μCT) analysis and scanning electron microscopy (SEM) were used to examine mineral densities of enamel and dentin, as well as the architecture of the enamel in age-matched Clcn7+/+ (wild type; WT) and Clcn7−/− (mutant) littermates. The μCT whole head scans also allowed us to comprehensively examine calvariae and suture formation in ClC-7 deficient mice.
MATERIALS AND METHODS
Animal study approval
All methodologies and animal manipulations related to this study were approved by the University of Southern California’s (USC) Institutional Animal Care and Use Committee (IACUC).
Samples
Six heads from three age-matched male pairs of Clcn7+/+ and Clcn7−/− littermates at ages post-natal (PN) day 19, day 20, and day 21 (one WT and one mutant from each age), were obtained from Dr. Thomas Jentsch (Kornak et al., 2001) having been initially fixed in 10% formalin for 1–2 weeks and then kept in 70% ethanol. Despite the small sample size (n=3 mutants and n=3 controls) there were no sample outliers, and we were able to generate statistically significant data in all parameters examined and reported on.
MicroCT (μCT) analysis
All six mouse heads were scanned, and reconstructed on a SCANCO μCT 50 at the University of Southern California’s Molecular Imaging Center with acquisition settings of: 70kVp, 85uA, 6W, filter AL 0.5mm, and 10 micron isotropic resolution. Depending on the sample size, acquisitions proceeded for ~ 2 hours. The images were exported in dicom format from the SCANCO workstation, volume/surface rendered, enamel and dentin segmented, and their mean intensity calculated in all sections with AMIRA5.5.0. Data collected from the same genotype, Clcn7+/+ or Clcn7−/−, were considered as a group. Student’s t-test was used for statistical analyses.
Scanning Electron Microscopy (SEM)
First, mandibular incisors were dissected and air-dried overnight. About 50 ml of hard EPON embedding material was thoroughly mixed with 25.7ml of resin, 9.3 ml of DDSA, 16.5 ml of NMA, and 0.8 ml of DMP-30 in a 50-ml conical tube within a vented fume hood. The EPON components came from a PELCO Eponate 12™ kit with DMP-30 (Catalogue # 18010, Redding, CA). Next, the EPON embedding mixture was diluted with propylene oxide by 30%, 60%, and 100% and mixed with the incisors for 1 hour sequentially to facilitate infiltration. The embedding EPON was deposited into a vacuum desiccator to eliminate bubbles and then poured into a rubber embedding mold holder topfull. The incisors were added and cured at 60–65°C for 36–48 hours.
Embedding blocks were first ground and polished with course sandpaper to expose the prismatic structure in a sagittal section of the incisors, the surface point of interest, and then continually milled with progressively finer grades of sandpaper down to 2.5-micron silicon carbide (P4000). (Lightly scraping the incisors with a toothbrush was necessary to remove debris during this process.) After cleaning with running water and a toothbrush, the block was then air-dried and acid etched with 1% nitric acid for 45 seconds, followed by a second water rinsing and air-drying. The air-dried sample was then mounted on a SEM stub with double sticky tape. Conductive paint (Colloidal silver liquid, Pelco TED PELLA Inc.) was applied to the whole stub which was then sputter coated with gold/platinum. JEOL JSM 6390LV was used for observation.
Sampling
All 3 wild type animals (ages 19–21) had a similar phenotype as observed by both μCT and SEM. All 3 mutant animals had a similar phenotype as observed by both μCT and SEM. Images presented are from the 21 day old mice (PN21).
RESULTS
Dentition of the mutant mice
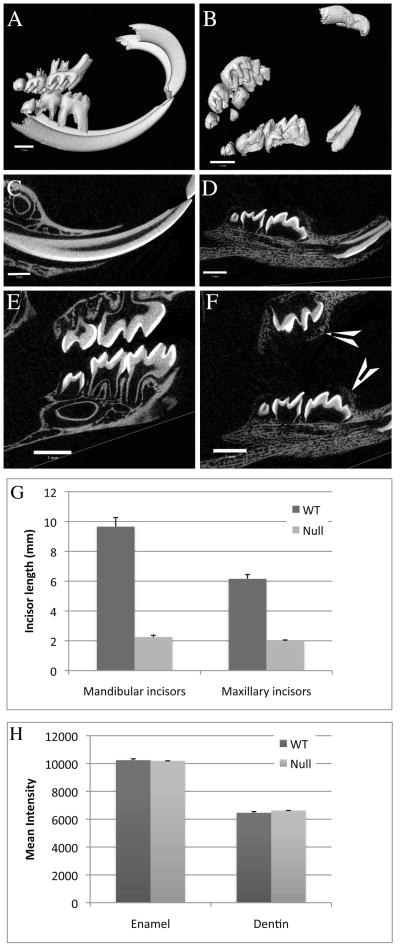
Three-dimensional μCT images showed teeth in Clcn7−/− animals that were mal-aligned (occlusal plan was disrupted) (Figure 1, panel B compared to Figure 1, panel A), which is likely explained by the fact that all molars remained unerupted at 3 weeks. The incisor teeth of the mutant mice were significantly smaller and distorted in shape compared to those of their wild type littermates (Figure 1, panel B compared to Figure 1, panel A; and panel G). The molar teeth of the mutant mice were absent of any root development as illustrated in Figure 1F when compared to the wild type (Figure 1E). Cross sectional slices (Figure 1, panels C–F) showed that the enamel and dentin densities of the mutant mice were comparable to those of the wild type mice. This result was further confirmed by quantitatively comparing the relative mineral density of all slices (indicated by mean intensity in Figure 1H). The density of enamel and dentin from post-natal (PN) day 19 to PN21 mice showed little change. The data collected from mice of the same genotype at three different time points (PN19, PN20, and PN21) were considered as a group. Neither enamel nor dentin showed any statistically significant difference in density between Clcn7+/+ and Clcn7−/− mice.
Figure 1.
Clcn7+/+ (panels A, C, and E) and Clcn7−/− (panels B, D, and F) teeth at age PN21. Panels A–F: μCT three-dimensional images (panels A and B) and cross-sectional slices of incisors (panel C and D) and molars (panel E and F) of two typical examples of teeth from Clcn7+/+ and Clcn7−/− littermates. AMIRA software was used to analyze the μCT data. Scale bars (1mm) are included in each panel. Arrowheads show molars that are encased in alveolar bones (panel F). Panel G: the length of mandibular and maxillary incisors. The average and standard deviations (error bars) were plotted using six incisors from three mice of the same genotype as a group. One-tailed paired Student’s t tests were run and the length of both mandibular and maxillary incisors in Clcn7−/− mice was significantly smaller (p<0.001) than that in Clcn7+/+ mice. Panel H: the density of mature enamel and dentin, indicated by mean intensity value from μCT scanning, were compared between Clcn7+/+ and Clcn7−/− mice using all three age matched pairs of mice. The averages and standard deviations (error bars) were plotted. Two-tailed paired Student’s t tests were run and no statistically significant difference in either enamel or dentin density was identified between Clcn7+/+ and Clcn7−/− mice.
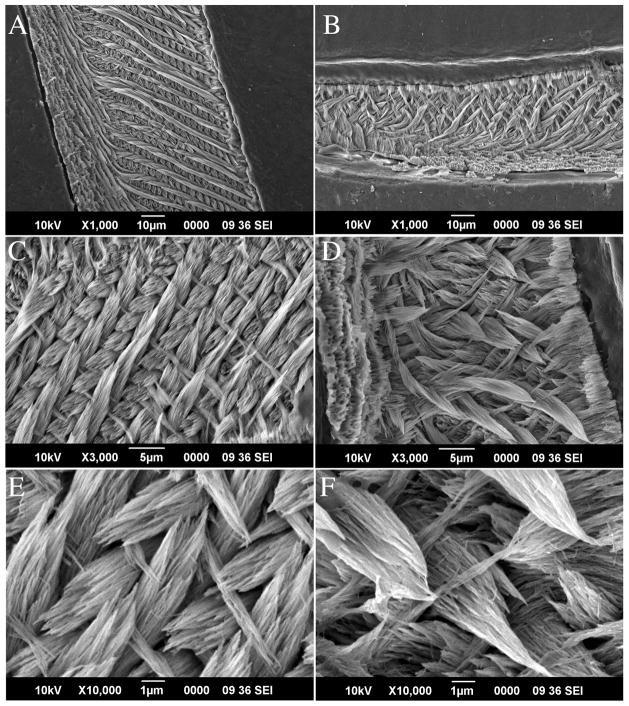
The architecture of the enamel was also examined using SEM. The mature portion of the enamel was noticeably thinner (hypoplastic) in Clcn7−/− incisors when compared to wild type littermate (~ half the thickness; Figure 2, panel B when compared to panel A). However, when the mature ends of the incisors were observed at higher magnification (Figure 2, panels C and D), the typical enamel rod-interrod organization seen in Clcn7+/+mice was also clearly apparent in the Clcn7−/− incisors. The enamel crystallites also appeared to be of normal morphology and size in Clcn7−/− incisors (Figure 2, panels E and F). Together with μCT data, these results suggest that the morphology and tooth growth were impacted by the lack of ClC-7 (most notably the lack of molar root formation and the size of the incisor teeth); however, the enamel prismatic structure (rod/interrod architecture) was not seemingly disrupted.
Figure 2.
Scanning electron microscope (SEM) images of tooth enamel. Teeth were embedded in EPON and ground and polished to expose the prismatic structure in a sagittal section of the incisors. Panels A, C and E are images from an incisor of Clcn7+/+ at age PN21 with 1000×, 3000×, and 10000× magnification, respectively, whereas Panels B, D and F are from the PN21 Clcn7−/− littermate using the same magnification scales. All panels show the mature end of the enamel structure.
Calvaria of the mutant mice
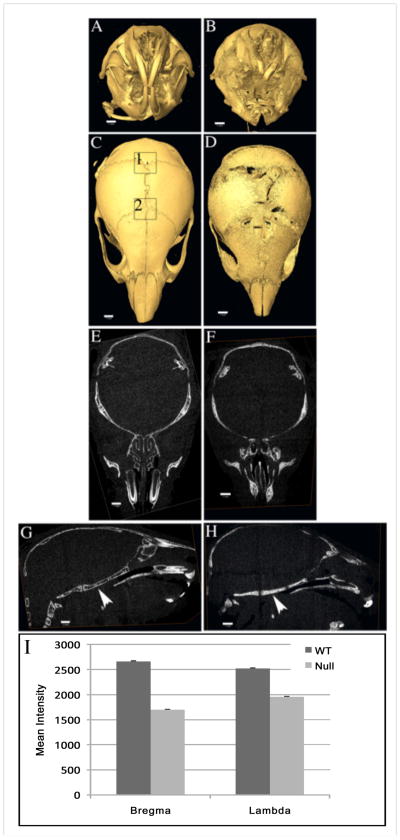
The whole head was scanned using μCT with the intent to document any dental abnormalities, however we were struck by some of the abnormal features apparent in the calvarial bones of the mutant mice. We observed that the cranial sutures were wide open (Figure 3, panel D compared to Figure 3, panel C). The bone density of area (4mm-square) surrounding bregma and lambda was quantified by measuring mean intensity for all six samples (indicated by mean intensity in Figure 3I). Clcn7−/− mice showed statistically significantly (p<0.001) decreased bone density at bregma and lambda compared to their littermates. (The open sutures in Clcn7−/− mice have not been previously reported.) While an impaired ability of osteoclasts to reabsorb bone in Clcn7−/− mice may account for an osteopetrosis phenotype, failure of cranial suture closure clearly indicated that there was decreased bone growth at these sites, suggesting the bone formation by osteoblasts was compromised as a result of impaired osteoclast function.
Figure 3.
Micro-CT images of Clcn7+/+ (panels A, C, E and G) and Clcn7−/− (panels B, D, F, and G) skulls at age PN21. Images of front view (panels A and B) and top view (panels C and D), and slices of transverse sections (panels E and F) and sagittal sections (panels G and H) are illustrated. AMIRA software was used to analyze μCT data. Scale bars (1mm) are included in each panel. Arrowheads in panels G and H show lack of bone marrow cavity at skull base in the mutant mouse compared to its wild type littermate. Bone density of areas surrounding bregma (2) and lambda (1) shown as 2mm squares in panel C were quantified. The corresponding areas in panel D (not shown) were similarly quantified. The mean intensity were plotted in panel I.
The surface of calvarial bones in Clcn7−/− mice was rough and porous (Figure 3 panel D compared to panel C). This was also apparent in the sagittal section of mandible (Figure 1, panel D compared to panel C), which showed that instead of being covered by more dense and compact bone, the surface of alveolar bone had the characteristics of trabecular bone. This observation was consistent with previous studies on the long bones that showed ClC-7 deficient mice lacked compact cortical bone development (Neutzsky-Wulff et al., 2010). In addition, μCT slices in transverse and sagittal section showed an increase in density of the cranial bones with the almost complete obliteration of bone marrow space (Figure 3, panels F and H when compared to panels E and G). Increased bone density in the mutant mice was especially noted at the skull base (Figure 3, panel H), where the bone marrow cavity shown in wild type mice (Figure 3, panel G) was almost completely occupied by irregular bone speculae in the mutant mice (Figure 3, panel G).
DISCUSSION
Mice lacking ClC-7 or its obligate β subunit, Ostm1, show severe osteopetrosis as well as lysosomal storage disease (Kornak et al., 2001; Kasper et al., 2005; Lange et al., 2006; Weinert et al., 2014). Without ClC-7/Ostm1 to operate alongside the V-ATPase to dissipate the transmembrane electrical potential difference, osteoclasts fail to acidify the extracellular resorption lacuna (Kornak et al., 2001). Lysosomal pH, on the other hand, is normal in cells lacking ClC-7/Ostm1 due to shunting provided by cation counterflux (Kasper et al., 2005; Steinberg et al., 2010). Knock-in mice, Clcn7unc/unc, in which ClC-7 Cl−/H+ exchange is converted to chloride channel, also display osteopetrosis (albeit milder) and lysosomal pathology, indicating that Cl−/H+ exchanging activity mediated by ClC-7, rather than Cl− conductance alone, is critical for osteoclast and lysosomal function (Weinert et al., 2010). In addition to Clcn7, most of the genes involved in osteopetrosis are associated with the pH regulation in osteoclasts including: carbonic anhydrase II (CAII), which generates a proton and a bicarbonate ion from carbon dioxide and water; anion exchanger AE2, which removes bicarbonate from the cytoplasm by exchange with chloride; and the V-ATPase subunit ATPV0A3 (TCIRG1), which transports protons (Del Fattore et al., 2008; Sobacchi et al., 2013). In many cases of osteopetrotic animals and human patients, the absence of teeth, or severe delay in tooth eruption, is a characteristic feature and often the first sign of osteopetrosis (Van Wesenbeeck and Van Hul, 2005). Osteopetrotic cattle with a Clcn7 mutation display gross gingival hamartomas, also likely linked to non-erupting teeth (Sartelet et al., 2014). Abnormal tooth eruption has been attributed to the deficiency of osteoclasts and subsequent defect in alveolar bone resorption during tooth development (Van Wesenbeeck and Van Hul, 2005).
Ameloblasts, like osteoclasts, express V-ATPase (Lin et al., 1994; Josephsen et al., 2010; Damkier et al., 2014). The pH at the mature end of enamel fluctuates between acidic and neutral and the pH regulation is important in enamel formation (Smith, 1998). Therefore we intended to examine whether ClC-7 plays a role in amelogenesis using the Clcn7 knockout mouse model. In this investigation, we found that at 19–21 days of age the molar and incisor teeth of Clcn7−/− mice had failed to erupt as previously reported (Kornak et al., 2001) and that the molar teeth of Clcn7−/− mice had no evidence of root formation. In the mutant mice the shape and size of continuously erupting incisors were even more severely affected than molars, and the enamel thickness of the incisors was approximately 50% less than the wild type. Despite these abnormalities, when examining the enamel and dentin, we found no marked difference in either mineral density content or the prismatic architecture and crystallite structural organization in the mutant mice when compared to wild type mice. These data suggest that although ClC-7 is ubiquitously expressed in most cells, including ameloblasts (Lacruz et al., 2013), the lack of ClC-7 expression in ameloblasts does not grossly impact the function of ameloblasts in directing enamel formation. The chloride channel (CLC) gene family encodes four chloride channels and five 2Cl−/H+ exchangers in mammals (Jentsch, 2008; Stauber et al., 2012); and it is feasible that the lacking of ClC-7 conductance in ameloblasts might be carried out (or substituted) by other CLC family members or other channels as observed in lysosomes where a cation conductance suffices for providing electric shunt for acidification by V-ATPase (Steinberg et al., 2010; Weinert et al., 2010). Therefore, observed defects in tooth development of ClC-7 deficient mice are most likely due to abnormal tooth eruption. Consistent with this idea, the incisors, with their continuously erupting nature, are affected much more than molars. A similar dental phenotype has been shown in another osteopetrosis animal model; the Ae2 knockout (Ae2−/−) mice (Josephsen et al., 2009). In the Ae2−/− mice the development of both the upper and lower incisors are impaired, however the authors noted that in these Ae2−/− mice the molar teeth had a radio-density comparable to that of wild-type (Josephsen et al., 2009).
With μCT, we noted that cranial sutures remained open at age 21 days, in contrast to the closed sutures seen in the wild type littermates. It seemed paradoxical that osteopetrosis, which is the result of bone overgrowth, and patent sutures, which are indicative of less than normal bone growth, were occurring in the same organism. These contradictory features could be due to differences in various skeletal sites with respect to osteoclastic bone digestion (Everts et al., 2009). This phenomenon is not unique for the Clcn7−/− mice. Pycnodysostosis, a rare autosomal recessive disease caused by the absence of active cathepsin K, an enzyme involving degrading the organic matrix of bones, shows both osteopetrosis in long bones and patent cranial sutures (Chen et al., 2007). Ae2a,b-deficient mice, deficient in the main isoforms of anion exchanger 2, which is responsible to keep near-neutral intracellular pH during proton pumping in osteoclasts, exhibit osteopetrosis of long bones but no alterations of calvaria (Jansen et al., 2009). Our observation in calvariae of the Clcn7−/− mice provided yet another example of bone-site-specific differences in bone cells leading to local differences in bone remodeling (Everts et al., 2009). Deficiency in osteoclast produces unbalanced bone remodeling affecting bone growth in sutures and long bones differently.
Acknowledgments
We thank Dr. T. J. Jentsch and Dr. T. Stauber for providing ClC-7 KO mice and for critical reading of the manuscript. The authors also thank the anonymous reviewers for their comments and critique. This work was supported by grant DE019629 from the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Abbreviations
- SEM
scanning electron microscopy
- RA
ruffle-ended ameloblasts
- WT
wild type
- DDSA
dodecenyl succinic anhydride
- NMA
nadic methyl anhydride
- DMP-30
dimethyl aminomethyl phenol
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Contributor Information
Xin WEN, Email: xwen@usc.edu.
Rodrigo S. LACRUZ, Email: rsl10@nyu.edu.
Michael L. PAINE, Email: paine@usc.edu.
References
- Brandt S, Jentsch TJ. ClC-6 and ClC-7 are two novel broadly expressed members of the CLC chloride channel family. FEBS Lett. 1995;377:15–20. doi: 10.1016/0014-5793(95)01298-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Yang S, Abe Y, Li M, Wang Y, Shao J, Li E, Li YP. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet. 2007;16:410–423. doi: 10.1093/hmg/ddl474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet. 2001;10:2861–2867. doi: 10.1093/hmg/10.25.2861. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Josephsen K, Takano Y, Zahn D, Fejerskov O, Frische S. Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H(+)-secretion by ameloblasts and variations in enamel buffer characteristics. Bone. 2014;60:227–234. doi: 10.1016/j.bone.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19–29. doi: 10.1016/j.bone.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Duan X. Ion channels, channelopathies, and tooth formation. J Dent Res. 2014;93:117–125. doi: 10.1177/0022034513507066. [DOI] [PubMed] [Google Scholar]
- Everts V, de Vries TJ, Helfrich MH. Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim Biophys Acta. 2009;1792:757–765. doi: 10.1016/j.bbadis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Jansen ID, Mardones P, Lecanda F, de Vries TJ, Recalde S, Hoeben KA, Schoenmaker T, Ravesloot JH, van Borren MM, van Eijden TM, Bronckers AL, Kellokumpu S, Medina JF, Everts V, Oude Elferink RP. Ae2(a,b)-deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEB J. 2009;23:3470–3481. doi: 10.1096/fj.08-122598. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- Josephsen K, Praetorius J, Frische S, Gawenis LR, Kwon TH, Agre P, Nielsen S, Fejerskov O. Targeted disruption of the Cl−/HCO3− exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci U S A. 2009;106:1638–1641. doi: 10.1073/pnas.0811682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O. Ion transporters in secretory and cyclically modulating ameloblasts: a new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol. 2010;299:C1299–1307. doi: 10.1152/ajpcell.00218.2010. [DOI] [PubMed] [Google Scholar]
- Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poet M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005;24:1079–1091. doi: 10.1038/sj.emboj.7600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Brookes SJ, Wen X, Jimenez JM, Vikman S, Hu P, White SN, Lyngstadaas SP, Okamoto CT, Smith CE, Paine ML. Adaptor protein complex 2-mediated, clathrin-dependent endocytosis, and related gene activities, are a prominent feature during maturation stage amelogenesis. J Bone Miner Res. 2013;28:672–687. doi: 10.1002/jbmr.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440:220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- Leisle L, Ludwig CF, Wagner FA, Jentsch TJ, Stauber T. ClC-7 is a slowly voltage-gated 2Cl(−)/1H(+)-exchanger and requires Ostm1 for transport activity. EMBO J. 2011;30:2140–2152. doi: 10.1038/emboj.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HM, Nakamura H, Noda T, Ozawa H. Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int. 1994;55:38–45. doi: 10.1007/BF00310167. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Rubinstein JL, Gruber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta. 2014;1837:857–879. doi: 10.1016/j.bbabio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Neutzsky-Wulff AV, Sims NA, Supanchart C, Kornak U, Felsenberg D, Poulton IJ, Martin TJ, Karsdal MA, Henriksen K. Severe developmental bone phenotype in ClC-7 deficient mice. Dev Biol. 2010;344:1001–1010. doi: 10.1016/j.ydbio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Sartelet A, Stauber T, Coppieters W, Ludwig CF, Fasquelle C, Druet T, Zhang Z, Ahariz N, Cambisano N, Jentsch TJ, Charlier C. A missense mutation accelerating the gating of the lysosomal Cl−/H+-exchanger ClC-7/Ostm1 causes osteopetrosis with gingival hamartomas in cattle. Dis Model Mech. 2014;7:119–128. doi: 10.1242/dmm.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9:522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. doi: 10.1146/annurev-physiol-030212-183702. [DOI] [PubMed] [Google Scholar]
- Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol. 2012;2:1701–1744. doi: 10.1002/cphy.c110038. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, Grinstein S. A cation counterflux supports lysosomal acidification. J Cell Biol. 2010;189:1171–1186. doi: 10.1083/jcb.200911083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Van Hul W. Lessons from osteopetrotic mutations in animals: impact on our current understanding of osteoclast biology. Crit Rev Eukaryot Gene Expr. 2005;15:133–162. doi: 10.1615/critreveukaryotgeneexpr.v15.i2.40. [DOI] [PubMed] [Google Scholar]
- Wartosch L, Fuhrmann JC, Schweizer M, Stauber T, Jentsch TJ. Lysosomal degradation of endocytosed proteins depends on the chloride transport protein ClC-7. FASEB J. 2009;23:4056–4068. doi: 10.1096/fj.09-130880. [DOI] [PubMed] [Google Scholar]
- Weinert S, Jabs S, Hohensee S, Chan WL, Kornak U, Jentsch TJ. Transport activity and presence of ClC-7/Ostm1 complex account for different cellular functions. EMBO Rep. 2014;15:784–791. doi: 10.15252/embr.201438553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, Rademann J, Stauber T, Kornak U, Jentsch TJ. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl− accumulation. Science. 2010;328:1401–1403. doi: 10.1126/science.1188072. [DOI] [PubMed] [Google Scholar]
- Xue Y, Wang W, Mao T, Duan X. Report of two Chinese patients suffering from CLCN7-related osteopetrosis and root dysplasia. J Craniomaxillofac Surg. 2012;40:416–420. doi: 10.1016/j.jcms.2011.07.014. [DOI] [PubMed] [Google Scholar]