Fig. 6.
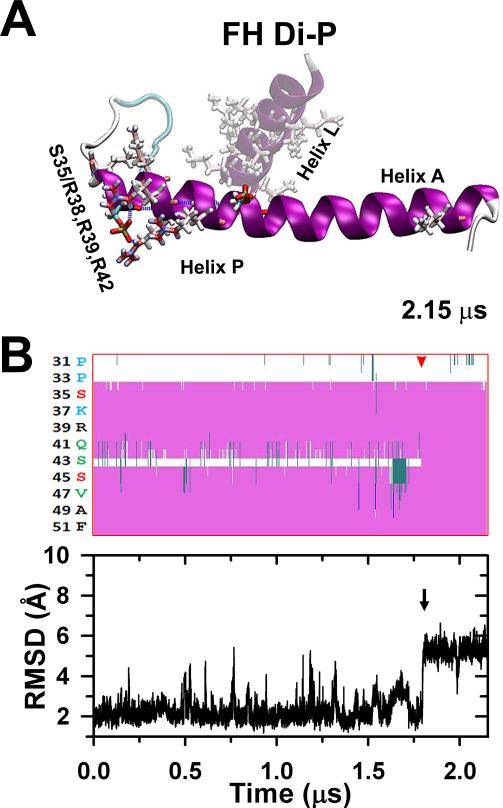
The stabilized FH pSer35 less compact peptide (Fig. 5A) was phosphorylated at Ser45, stabilized after 2.15 μs to a full elongated conformation in which the rest of helix P gets aligned along helix A culminating the disorder-to-order transition.30 The diphosphorylation does not establish new salt bridges (A). The secondary structure evolution of the stabilized Ser35 monophosphorylated peptide after Ser45 diphosphorylation is shown in (B, top) indicating that after ~1.8 μs (red arrowhead) the Ser35 and Ser45 parts of helix P become a continuous helix along helix A. The black arrow corresponds to the last conformational change that stabilizes the peptide correlated to the RMSD evolution (B, bottom).