Figure 2.2.
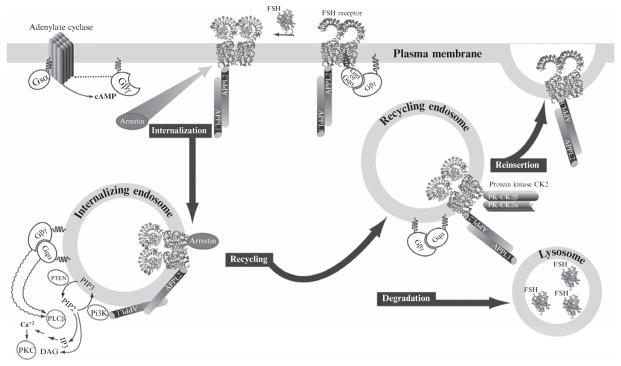
Downward traffic of the FSHR is linked to intracellular signaling pathways. FSH binding to FSHR induces the exchange of GDP from the stimulatory subunit (Gαs) of the G protein heterotrimer. Upon binding of GTP, the G protein heterotrimer dissociates and Gαs activates adenylate cyclase, while the βγ dimer activates phospholipase Cβ. These are the canonical pathways for FSH activation. The receptor iL1 and iL3 are subject to phosphorylation by GRKs and subsequent binding of arrestins. Internalization via arrestins likely follows the same early endosome pathway as that of APPL proteins; the latter interacts constitutively with the iL1 of the FSHR. Mutation of K376A in FSHR prevents association of APPL1 with FSHR and loss of FSH-induced intracellular calcium release and inositol triphosphate (IP3) production, with no effect on internalization. Knockdown of arrestin results in a loss of FSH-stimulated ERK phosphorylation. FSHR is ubiquitinated, likely enabling its sorting into recycling vesicles with bound FSH or fused with lysosomes where unliganded FSH is degraded. Recycled unoccupied receptor in theory can engage FSH for another round of FSH stimulation.
