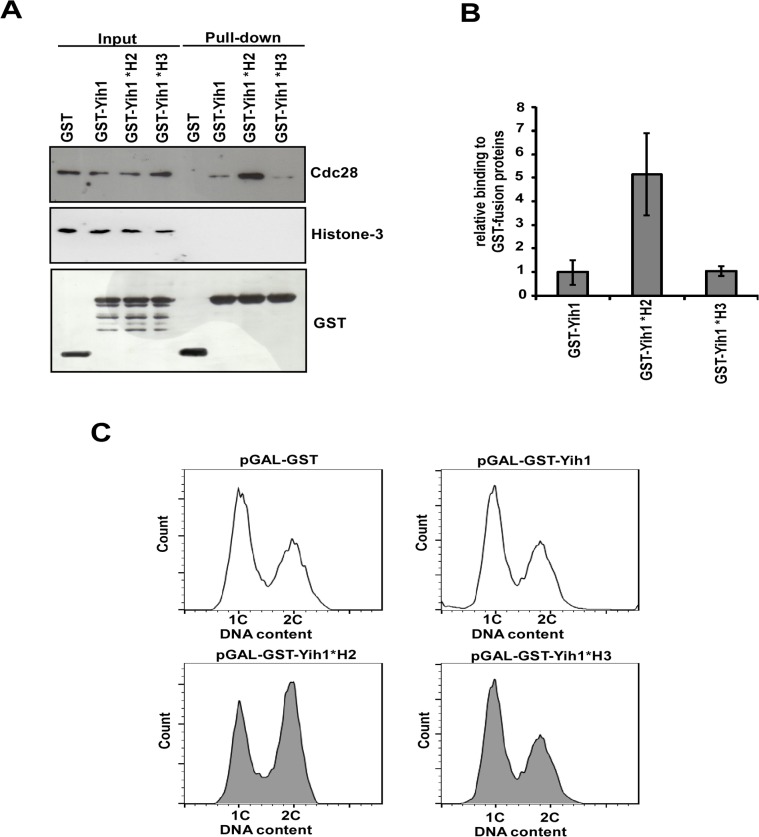
Fig 10. Effect of Yih1 RWD domain amino acid substitutions on Cdc28 binding.
(A) In vivo GST-pull-down assays were performed on yih1Δ strains (MSY-Y2) expressing the GST-Yih1 fusion proteins as indicated, or GST alone, from a galactose inducible promoter. *H2 depicts E87A and D90A substitutions and *H3 depicts D102A E106A substitutions. Cells were grown to log-phase and harvested. Equal amounts of WCEs (2 mg) were subjected to glutathione-mediated GST pull-down assays. The precipitates (100% of the bound proteins – right) and the input (1/100th of the input – left) were assessed by immunoblot to detect the indicated proteins. One representative blot from three independent experiments is shown. (B) Amount of endogenous Cdc28 bound to the GST-Yih1 fusion proteins was determined from (A) by dividing the signal intensity of Cdc28 by the precipitated amount of the respective GST-Yih1 fusion proteins. Data represent means and S.E. of three independent experiments. (C) Representative flow cytometry histograms showing the DNA content of wild type cells (MSY-WT2) expressing GST alone, GST-Yih1 (unfilled histograms), GST-Yih1*H2 or GST-Yih1*H3 (gray filled histograms) from a galactose inducible promoter. The distribution of cells in G1 (1C), S and G2/M (2C) is shown.