Summary
Behaviors and underlying brain circuits show characteristic changes across the life-span that produce sensitive windows of vulnerability and resilience to psychopathology. Understanding the developmental course of these changes may inform which treatments are best at what ages. Focusing on behavioral domains and neurobiological substrates conserved from mouse to human supports reciprocal hypothesis generation and testing that leverages the strengths of each system in understanding their development. Introducing human genetic variants into mice can further define effects of individual variation on normative development, how they contribute to risk and resilience for mental illness, and inform personalized treatment opportunities. This article emphasizes the period of adolescence, when there is a peak in the emergence of mental illness, in particular, anxiety disorders. We present cross-species studies relating fear learning to anxiety across development, and discuss how clinical treatments can be optimized for individuals and targeted to the biological states of the developing brain.
Neurodevelopmental Framework
The brain is an extremely dynamic organ displaying dramatic differences in gene expression, neurogenesis, neural circuit formation and maturation, and behavior across the life span (Lee et al., 2014). Developmental changes in brain and behavior have evolved to facilitate completion of stage-specific prerogatives: forming nurturing bonds in infancy, juvenile exploration of the environment, and the formation and maintenance of stable relationships in adulthood. Adaptive changes across normative development can lead to imbalances that predispose to or protect from mental illness in interaction with individual genetic factors and environmental exposures. Enhancing clinical outcomes requires an appreciation of neurodevelopmental changes in brain and behavior across diverse behavioral domains and treating the individual by targeting their developmental strengths.
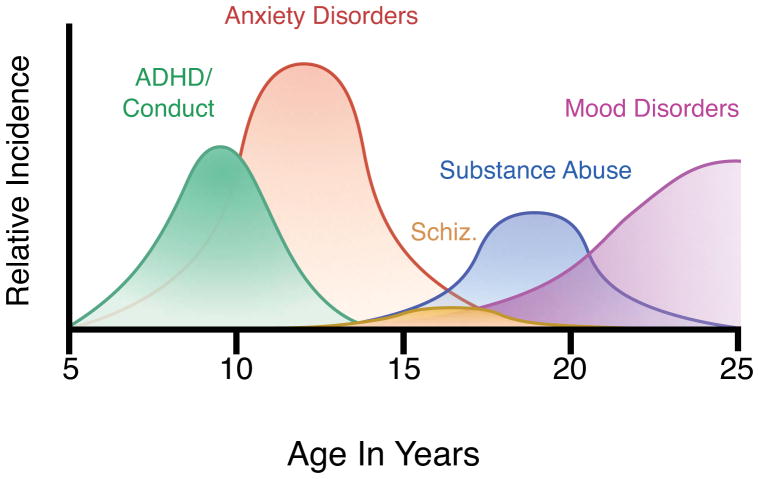
Our neurodevelopmental framework considers three interrelated concepts that inform the distinct peaks in incidence of different disorders: developmental trajectory, dynamic interaction of systems, and sensitive periods (Casey et al., 2014). Developmental trajectory refers to the course of brain and behavioral changes over time. Behavioral domains and their neural substrates display different developmental profiles and these normative courses must be defined to understand how the relative strengths and liabilities of different developmental stages contribute to the characteristic age of onset of different disorders and, in particular, the high incidence of mental illness in adolescence (Figure 1).
Figure 1. Developmental emergence of mental disorders.
Based on data from (Kessler et al., 2005; Kessler and Wang, 2008)
Behavior results from the coordinated activity of diverse neural structures through complex neural circuits. Dynamic interaction of systems refers to the fact that circuit function and behavior will vary across time as different components of neural circuits mature according to different trajectories. As structures and the tracts connecting them mature at different rates, relative imbalances can occur, particularly during adolescence when emotional behaviors are biased towards subcortical drive due to the late maturation of prefrontal cortex (Casey et al., 2008; Gogtay et al., 2004). These developmental imbalances are adaptive but create vulnerabilities that, when exacerbated by biological, environmental, and genetic factors, give rise to a cascade of more complex deficits as subsequent brain regions mature and interact with a dysregulated system (Masten and Cicchetti, 2010).
Finally, neural and behavioral plasticity is not fixed. During development there are temporally limited “sensitive periods” of heightened plasticity during which different neural systems and behavior are particularly receptive to different types of experience. The visual system displays a very distinct sensitive or critical period for stimulus-induced plasticity providing an opportunity to define the mechanisms of transient plasticity and identify methods to manipulate it (Hensch, 2004; Knudsen, 2004). The systems regulating fear and anxiety also display “sensitive periods” (Nabel and Morishita, 2013) and these times of enhanced plasticity create vulnerability to pathogenic experiences and provide windows of opportunity when therapeutic interventions may be particularly successful (Lee et al., 2014).
Development of fear and anxiety
As anxiety disorders are the most common psychiatric illnesses in youth, affecting as many as 1 in 10, and these diagnoses peak in adolescence (Kessler et al., 2005), we focus this article on understanding and treating these disorders from a neurodevelopmental perspective. A core feature of anxiety disorders is difficulty identifying when situations that have been experienced as threatening in the past are currently safe. Based on principles of fear extinction learning, exposure-based cognitive behavioral therapy (CBT) focuses on desensitization through repeated exposures to fearful triggers in a safe context. This process relies upon frontoamygdala circuitry that undergoes substantial neurodevelopmental changes from childhood to adulthood (Gee et al., 2013; Hare et al., 2008) and likely contributes to both developmental and individual differences in anxiety and its treatment. In this context, 40–50% of youth with anxiety disorders do not respond to exposure based CBT treatment (Walkup et al., 2008). Rodent and human studies suggest that fear extinction learning is diminished during adolescence (Kim et al., 2011; McCallum et al., 2010; Pattwell et al., 2012b) relative to childhood and adulthood, and that this diminished extinction stems from altered neuroplasticity in prefrontal cortex in adolescents (Pattwell et al., 2012b). Moreover, alterations in sensitive periods may contribute to the onset of anxiety, constraining the flexibility and repertoire of mechanisms for fear reduction among adolescents with anxiety disorders. In this article, we highlight how changes in brain development during the transitions into and out of adolescence and individual genetic variation impact the capacity for emotion regulation and dysregulation in anxiety disorders. Based on these findings, we provide a framework for using age and genetics to devise novel therapeutic and preventative strategies to maximize effectiveness in treating these disorders.
Neural circuits for fear learning and regulation
A core feature of anxiety disorders is difficulty learning which cues and contexts signal safety and which signal a threat (Charney and Manji, 2004; Duman et al., 1997; Nestler et al., 2002; Pine, 2007). Fear learning involves making associations between previously experienced negative events and the cues and contexts that predicted their occurrence. Experimental studies typically model this associative learning using Pavlovian conditioning paradigms, in which a neutral cue is paired with an intrinsically aversive stimulus. This pairing produces a learned association between the previously neutral cue, now the conditioned stimulus (CS), and the aversive unconditioned stimulus (US), which enables the CS to elicit a range of physiological and behavioral conditioned responses (CRs) to the anticipated threat. In experimental studies of rodents, the most typical CR assessed is freezing. In humans, common CRs include changes in skin conductance, startle responses, and pupil dilation. Although learned fear memories are persistent, their behavioral expression can be inhibited through new learning that a once threatening stimulus is now safe. Experimentally, this process of extinction learning is modeled by repeatedly presenting the CS without the aversive US and is typically accompanied by a gradual decrease in the expression of the CR. The persistence of the original fear memory is evidenced by the fact that extinguished fear often returns under a number of circumstances including a change in context (renewal), exposure to an aversive stimulus (reinstatement), or the mere passage of time (spontaneous recovery) (Bouton, 2004).
The circuitry involved in fear regulation in the adult brain has been delineated in both human imaging and rodent studies (Davis and Whalen, 2001; LeDoux, 2000). The amygdala is the core structure involved in fear acquisition and expression of learned fear memories. In-depth anatomical, electrophysiological, and neuroimaging studies have detailed the various components of the amygdala and their roles in fear learning and memory. In standard auditory cued fear conditioning, projections from the cortex and thalamic nuclei receiving sensory inputs converge on the lateral nucleus of the amygdala (LA) simultaneously (Collins and Pare, 2000; Quirk et al., 1995; Sotres-Bayon et al., 2006). The LA, along with the basal nucleus of the amygdala (BA), serve as the primary fear-learning interface, and collectively integrate the relevant sensory information and relay it to the central nucleus (CE). Serving as the amygdala’s secondary interface, the CE elicits fear responses through downstream projections to hypothalamic and brain stem nuclei to engage autonomic responses (Maren, 2001). The cytoarchitecture of the amygdala is complex: the basolateral amygdala (BLA) contains primarily glutamatergic projection neurons, while the medial CE contains primarily GABAergic neurons with medium spiny neuronal morphology (Ehrlich et al., 2009). During fear acquisition, projections from the hippocampus (specifically the CA1 region) also provide information to the BA about the surrounding environment (Bouton et al., 2006). Hippocampal–BA integration of contextual information about the surrounding environment has a major influence on downstream CE activity and subsequent fear responses (Fanselow and Dong, 2010; Maren, 2001).
During fear extinction learning, prefrontal cortical regions are important for appropriately adjusting behaviors when the emotional significance of a given cue changes (Sotres-Bayon et al., 2006). The ventromedial prefrontal cortex (vmPFC), in particular, has been shown to be important for making the switch from fear expression to fear suppression during fear extinction learning and retention (Milad and Quirk, 2002; Pare et al., 2004; Santini et al., 2004). Distinct subregions within the vmPFC have been differentially implicated in the expression and extinction of conditioned fear (Santini et al., 2008; Sierra-Mercado et al., 2011; Sotres-Bayon and Quirk, 2010). Specifically, the dorsally located prelimbic cortex (PL) is associated with production of conditioned fear responses and expression of conditioned fear behaviors (Corcoran and Quirk, 2007) whereas the more ventrally located infralimbic cortex (IL) is associated with suppression of conditioned fear responses (Burgos-Robles et al., 2009; Hefner et al., 2008; Knapska and Maren, 2009). The infralimbic cortex can dampen fear responses via projections to a cluster of inhibitory intercalated cells located within the amygdala. These inhibitory intercalated cells modulate activity in the central nucleus, thereby suppressing the CE output, and dampening downstream physiological processes associated with the fear response (Berretta et al., 2005; Likhtik et al., 2008).
The hippocampus also plays a critical role in fear extinction, as it modulates frontoamygdala function by supplying contextual information about the degree of threat or safety in the environment (Fanselow and Dong, 2010; Orsini and Maren, 2012), which is accomplished via projections from ventral CA1 hippocampus to the BA (Fanselow and Dong, 2010; Orsini et al., 2011). Whether experienced as safe or threatening, these hippocampal-BA connections can modulate subsequent fear responses via projections to the CE. Connections between the ventral CA1 hippocampus and vmPFC (IL) modulate extinction learning by detecting contextual cues in the surrounding environment (Hugues and Garcia, 2007; Rosas-Vidal et al., 2014; Sierra-Mercado et al., 2011). In addition, recent, studies in awake behaving rats show that the ventral CA1 also directly inhibits PL activity only after extinction training, presumably by activating interneurons in the PL (Sotres-Bayon et al., 2012). In sum, the neural circuitry underlying fear expression and regulation involves complex interactions among frontolimbic brain circuitry and variation in the connectivity of this circuitry may impact its function.
Sensitive periods for fear learning and regulation
Although multiple studies across species have delineated a fairly detailed model of the neurocircuitry supporting fear learning and extinction in adulthood, there has been less focus on the neurodevelopment of these functional circuits. The prefrontal and subcortical circuitry implicated in adult fear learning undergoes substantial developmental change from childhood through adulthood (Gogtay et al., 2004; Lenroot and Giedd, 2006; Raznahan et al., 2014; Sowell et al., 1999). Mirroring these pronounced changes in the brain, numerous studies to date suggest that fear learning and regulation exhibit qualitative changes across development.
Infantile sensitive period for fear learning
In rodents, fear learning emerges early in postnatal development and is linked to the maturation of the amygdala (Landers and Sullivan, 2012). Prior to postnatal day 10 (P10), infant rats exhibit a paradoxical approach response to an odor stimulus previously paired with shock (Camp and Rudy, 1988; Sullivan et al., 2000). This early postnatal period corresponds to a sensitive period for attachment learning, and the suppression of fear responding during this period may functionally promote attachment between the infant and caregiver, even if the quality of care received is poor (Landers and Sullivan, 2012). After P10, the odor-shock conditioning produces a conditioned odor aversion, reflecting the emergence of cued fear learning. This behavioral change coincides with the onset of learning-induced synaptic plasticity within the amygdala (Thompson et al., 2008). However, P12-P15 rodent pups exhibit persistent odor preference when paired with a shock in the presence of the mother, but odor aversion in the absence of the mother, as maternal presence led to suppression of pup corticosterone and its regulation of amygdala activity (Moriceau Sullivan 2006). Even when animals have developed the ability to learn conditioned aversion, early fear memories remain qualitatively different from those of adults in that they are not as persistent. Conditioned fear learned at P18 appear to be forgotten within ten days (Campbell and Spear, 1972; Kim and Richardson, 2007). Conversely, fear memories conditioned at P23 do not degrade, but are highly susceptible to interference, showing persistent attenuation following extinction learning, a pattern not seen in older animals (Pattwell et al., 2012b). Notably, this “infantile amnesia” for fear memories is experience-dependent, and modulated by conditions of early-life stress. Animals that have experienced chronic maternal separation at P17 exhibit full recall of fear ten days later (Callaghan and Richardson, 2011). Contextual fear conditioning in rodents emerges later than cued fear learning (Akers et al., 2012; Rudy, 1993). Whereas P17 rats do not appear to extend learned fear associations to the broader surrounding environment, adult-like contextual fear conditioning emerges by P24. Recently it has been shown that neurogenic waves in the hippocampus in infant rodents prevent contextual fear memory persistence (Akers 2014). The emergence of contextual fear learning and memory may reflect increased maturation of the hippocampus and its connections to the amygdala (Raineki et al., 2010).
Juvenile sensitive period for fear extinction
As with acquisition and retrieval of fear learning, extinction learning also changes markedly across development especially during the transition through the juvenile period. Extinction training in pre-weanling animals (prior to P24) produces the typical decrease in fear expression. Unlike adult animals, these animals do not exhibit the fear re-emergence phenomena that typically occur following extinction training (Gogolla et al., 2009; Kim and Richardson, 2007; Yap and Richardson, 2007). This lack of spontaneous recovery, reinstatement, and renewal suggests that fear memories at this developmental stage are fragile or vulnerable to interference and may depend less on IL input (Kim and Richardson, 2008). However, as stated above, we have shown that in P23 rodents, memories do not degrade on their own, but rather are highly susceptible to interference and paralleled by IL potentiation during extinction learning (Pattwell et al., 2012b). Nonetheless both studies show persistent attenuation of the fear memory (e.g., lack of spontaneous recovery or reinstatement of fear memory), unlike in older rodents in which the recovery of fear typically occurs.
Developmental critical periods in the visual system have been related to maturation of the extracellular matrix surrounding fast-spiking GABAergic interneurons that express parvalbumin (PV). In particular, the formation of perineuronal nets (PNNs), an organized form of chondroitin sulfate proteoglycancontaining extracellular matrix, initiates critical period closure in the visual cortex (Berardi et al., 2003; Pizzorusso et al., 2002). A significant increase in PNN’s has been observed in the amygdala during the juvenile timeframe, which is the precise timeframe for the transition from the juvenile form of fragile fear memory, to the more adult-like state in which recovery of extinguished fear typically occurs (Gogolla et al., 2009). There are two possible mechanisms by which PNNs might prevent fear memory erasure. PNNs may stabilize fear memories by rendering potentiated synapses resistant to reversal of long-term potentiation, or PNN formation might give rise to changes in local GABA-mediated inhibition. The latter mechanism is plausible given that PNNs form primarily around parvalbumin-positive GABAergic interneurons and that GABAergic neurotransmission mediates several forms of BLA synaptic plasticity (Duvarci and Pare, 2014). Moreover, structural degradation of these PNNs in adulthood reintroduces a juvenile-like state in which extinction results in a persistent attenuation of fear memory (Gogolla et al., 2009).
In contrast to the ease with which fears are diminished in these younger animals, both fear extinction learning and retention are attenuated during adolescence (Kim et al., 2011; McCallum et al., 2010; Pattwell et al., 2012b) (Figure 2A). Relative to pre-and post-adolescent animals, adolescents exhibit diminished fear extinction learning that is paralleled by an absence of extinction-learning-induced plasticity within the IL (Pattwell et al., 2012b). Adolescent rats require either more extinction trials or a pharmacological intervention, such as the NMDA receptor modulator, d-cycloserine, to achieve reductions in fear expression comparable to younger or older rats (McCallum et al., 2010). This blunted fear extinction during adolescence is associated with a lack of activity in prefrontal cortex, specifically IL, as assessed by phospho-MAP kinase immunohistochemistry (Kim et al., 2011) or c-Fos immunohistochemistry (Pattwell et al., 2012b) compared to younger and older ages. Electrophysiological recordings at IL and PL synapses across development reveal that a fear-conditioning induced potentiation of PL synapses present in adult mice is absent in adolescent mice. Furthermore, extinction-induced enhancement of IL synaptic plasticity in adult mice, is lacking in adolescent mice (Pattwell et al., 2012b). These studies suggest that the development of cued fear extinction progresses in a nonlinear manner, with adolescents showing diminished extinction learning relative to preadolescents and adults.
Figure 2. Development of cued fear extinction parallels clinical response to cognitive behavioral therapy in anxiety disorders.
(A) Reduced fear extinction learning in both mice and human during adolescence (Pattwell et al., 2012b). (B) Diminished treatment response during adolescence based on data from the Child/Adolescent Anxiety Multimodal Study (CAMS) (Walkup et al., 2008) subdivided by developmental stage (A) adolescents show nonsignificant decrease in responsiveness to cognitive behavioral therapy (Drysdale et al., 2014). *Adolescent extinction is significantly lower than childhood and adulthood.
Adolescence is a time of exploration when one must leave the safety of his or her familial environment in order to attain reproductive success. As specific danger cues remain relevant during this novelty-seeking period, cued fear expression remains intact and is resistant to extinction during adolescence. The pronounced structural remodeling of subcortical-prefrontal connections (e.g. myelination, synaptic pruning) that occurs during adolescence is likely to contribute to these qualitative shifts in fear regulation (Somerville and Casey, 2010). For example, there is substantial pruning of neurons projecting from the IL to the basal amygdala from adolescence to adulthood (Cressman et al., 2010). Changes in connectivity between both the amygdala and the hippocampus, and the vmPFC during adolescence may initiate the shift from the restricted subcortical circuitry governing fear learning in juvenile stages, toward the more flexible and expansive circuit for fear regulation that is evident in adulthood.
Human studies of fear learning across development have been somewhat limited by the methodological constraints involved in designing effective aversive learning paradigms that are ethical to conduct in children. Typically, these paradigms use unconditioned stimuli such as white noise, unpleasant images, or a combination of the two (Casey et al., 2013; Shechner et al., 2014). As in rodents, fear extinction in humans is also selectively attenuated during adolescence relative to children and adults (Pattwell et al., 2012b) (Figure 2A). To date no functional imaging studies of fear extinction across development have been reported, but a recent fMRI study examining developmental changes in connectivity between the medial prefrontal cortex and the amygdala found that BOLD activity within the vmPFC and the amygdala begins to shift from a positive to negative correlation from childhood to adolescence that is then stabilized during adulthood (Gee et al., 2013). These functional connectivity measures provide an indication of the pronounced maturational changes in the dynamic interaction between these regions during adolescence.
In a remarkable contrast to cued fear associations, which appear particularly prominent during adolescence due to inefficient extinction learning, adolescents appear insensitive to contextual fear conditioning. Unlike juvenile and adult mice, adolescent mice returned to the context in which they experienced an aversive event do not display a fear response (Pattwell et al., 2011). This suppression of contextual fear during adolescence is due to a failure of contextual fear retrieval as opposed to acquisition because the same animals tested in early adulthood display a fear response to the context. This finding demonstrates that the developmental course of subdomains of behavior may be very different with implications for the development and treatment of psychopathology.
Impact of common polymorphisms across development
Although the genome is largely static across the life span, the phenotypic expression of genetic polymorphisms can vary during development (Casey et al., 2009). Common genetic polymorphisms act by imposing quantitative changes in gene function. The impact of these molecular effects on higher-level brain and behavioral phenomena may only be apparent when biochemical and neural systems that interact with polymorphic genes are at a critical developmental threshold. Common polymorphisms may act by altering developmental trajectories; shifting windows of risk and resilience to earlier or later ages or widening or constricting these developmental windows ultimately impacting risk for psychopathology and the efficacy of specific treatments.
Understanding the effects of common genetic variation on the development of different behavioral domains, and relating those changes to psychopathology, is valuable because common polymorphisms have such potential as biomarkers guiding personalized psychiatric medicine. However human behavioral genetic association studies are very challenging because human samples contain a great deal of heterogeneity in terms of their various life experiences and genetic background, which can confound genetic associations. Moreover, understanding the development of behavioral domains would require large numbers of subjects of a wide range of ages to be assessed.
To address the difficulties of human genetic association studies we have developed a strategy in which individual human polymorphisms are introduced into the genomes of inbred mice through genetic knock-in techniques (Glatt and Lee, 2015) (Figure 3). The resulting mice recapitulate the detailed phenotypic effects of the human variant allele and can be compared to wild type mice if they express the ancestral human allele. These mice can then be subjected to detailed, invasive, and controlled analysis to identify the effects of the polymorphism on phenotypes across the biological spectrum from molecule, to signaling pathway, neural and circuit function, ultimately to behavioral domains and human pathology-like behaviors such as exploratory behavior under threat. Polymorphic effects identified in mice can then be used to design, refine, interpret, and validate human genetic association studies. Leveraging cross-species translation and integration across multiple levels of analysis enhances the reliability and precision of behavioral genetic associations, a necessary first step in developing them as clinical biomarkers.
Figure 3. Cross-species approach to individual variation in behavior.
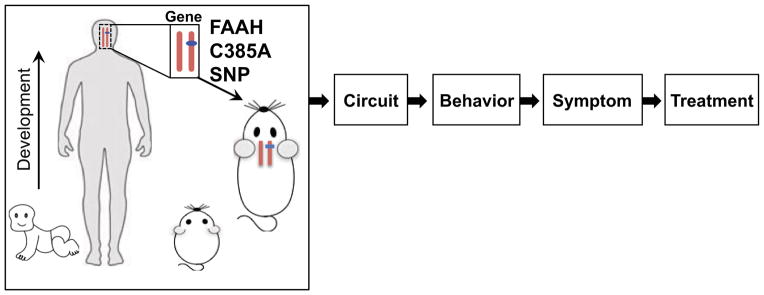
Introducing common genetic polymorphisms into the mouse genome supports parallel studies of brain structure and function using species-specific techniques across levels of biology. Polymorphic effects on conserved behaviors cross-validate and refine results from each species. Analysis of gene x development interactions can be identified in mice and specific hypotheses tested in humans.
Such humanized polymorphic mice greatly facilitate gene x development studies because large numbers of mice on identical genetic backgrounds can be studied at any number of developmental ages. Experiential variables such as stress can also be controlled and manipulated to ensure that environmental factors that might impact behavior and development are normalized across genotypes and can be studied systematically.
Knock-in mouse models of BDNF Val66Met and FAAH C385A
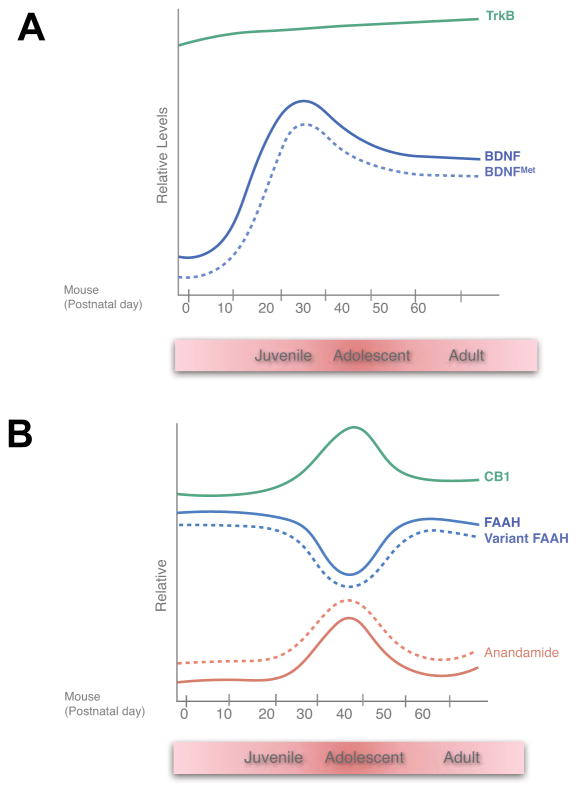
We have begun to implement this approach focusing on two common functional polymorphisms in genes that have central roles in major signaling systems in the brain that have been implicated in learning and memory, and fear memory processes in particular: Brain-Derived Neurotrophic Factor (BDNF) and Fatty Acid Amide Hydrolase (FAAH). These molecules display unique patterns of expression across development that may contribute to the developmental differences in attributes of fear memories and may also create windows where the effects of polymorphic variation are particularly pronounced or attenuated (Figure 4).
Figure 4. Developmental expression of (A) BDNF and (B) components of the endocannabinoid system in the brain.
Both systems peak in adolescence and may contribute to unique attributes of fear learning during this stage. Effects of BDNF Val66Met and FAAH C385A across development are presented in dotted lines.
BDNF is a growth factor acting through TrkB tyrosine kinase receptors to promote neuronal survival and differentiation. It is also critical for experience dependent synaptic plasticity and memory including fear learning (Andero and Ressler, 2012; Chao, 2003). Loss of BDNF expression in adult genetic knockout mice leads to impaired fear learning and increased anxiety-related behaviors (Chen et al., 2006). Clinically, serotonin selective reuptake inhibitor (SSRI)–induced increases in BDNF expression are required for their anxiolytic effects (Duman and Monteggia, 2006). Endogenous levels of BDNF in the brain rise dramatically starting at P10 in mice and peakbetween P20-P30 (Figure 4A), a time period, which corresponds with the transition from juvenile to adolescent forms of fear memories (Katoh-Semba et al., 2007; Kolbeck et al., 1999). The developmental coincidence of increased BDNF levels with the onset of inefficient extinction learning and retention is intriguing, because in adulthood BDNF has been established to play key roles in cued fear extinction (Peters et al., 2010). These BDNF developmental findings provide further evidence that extinction behavior in adolescence may be regulated by different signaling systems than those in adulthood. Human populations contain a common nonsynonymous polymorphism coding for the replacement of the conserved Valine 66 with a Methionine residue (BDNF Val66Met, rs6265). This substitution has been shown to lead to decreased activity dependent BDNF secretion in vitro. In adulthood, genetic knock-in of the variant Met allele causes decreased dendritic complexity, reduced hippocampal volume, and an SSRI-resistant increase in anxiety-related behaviors (Chen et al., 2006).
FAAH is the major catabolic enzyme for the endocannabinoid, anandamide (AEA), an agonist for CB1 receptors in the brain (Ahn et al., 2009). Reduced FAAH activity due to genetic knockout or pharmacologic inhibition knockout lead to dramatic increases in AEA in the brain accompanied by enhanced fear extinction learning and decreased anxiety-related behaviors. FAAH expression displays a transient decrease during adolescence with a trough of expression occurring between P30 and P40 (Figure 4B) slightly later than the BDNF peak between the juvenile to peri-adolescent timeframe (P2535) (Lee and Gorzalka, 2012; Lee et al., 2013) than the BDNF peak. AEA levels display inverse changes across development consistent with FAAH as its major catabolic mechanism. In addition, there is a concomitant transient increase in expression of CB1 receptors in cortical and subcortical regions in this same timeframe suggesting that there is an overall enhanced endocannabinoid signaling during adolescence. There is a common human C/A polymorphism at position 385 of the FAAH protein coding sequence (FAAH C385A, rs324420) that leads to replacement of a conserved Proline residue at position 129 with a Threonine, which destabilizes the FAAH protein leading to lower steady state levels of FAAH expression (Sipe et al., 2002) and, as we have recently demonstrated, increased AEA in the brain (Dincheva et al. 2015).
Parallel mouse humans studies of the BDNF Val66Met and FAAH C385A SNPs Fear extinction learning
The rich basic science literature relating BDNF and FAAH to cued fear extinction learning combined with understanding of the molecular phenotypes of BDNF Val66Met and FAAH C385A allowed us to form a priori hypotheses of how the polymorphisms would affect fear extinction learning. Specifically we hypothesized that the BDNF Met allele, by reducing BDNF signaling would reduce the efficiency of fear extinction learning and the FAAH A allele, by increasing levels of extinction-facilitating AEA, would enhance fear extinction learning.
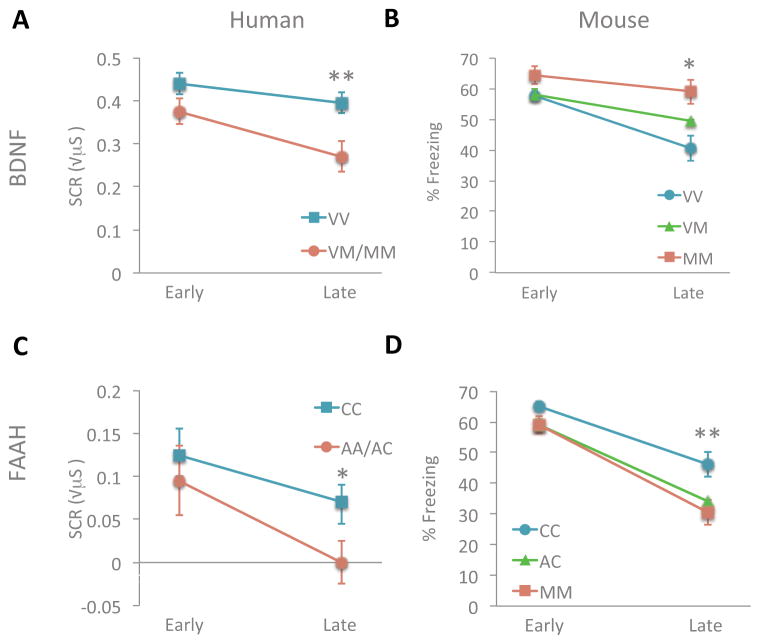
To test these hypotheses we conducted parallel studies in the polymorphic knock-in mice and human population samples using species-specific versions of fear conditioning paradigms. We confirmed our hypotheses for both genes in both model systems. The BDNF Met allele was associated with reduced fear extinction learning in human carriers (Met homozygotes and heterozygotes versus Val homozygotes) (Figure 5A). In mice, controlled breeding allows the generation of large numbers of animals of each genotype, which supported the identification of an additive effect of the Met allele (Soliman et al., 2010). That information can inform the analysis of future human association studies of BDNF Val66Met. The FAAH A allele was associated with enhanced fear extinction learning in human carriers and, in contrast to the BDNF Met allele, in mice the A allele appeared dominant causing similar enhancement extinction learning of heterozygotes and homozygotes (Dincheva et al., 2015) (Figure 5B).
Figure 5. Individual variation in cued-fear extinction learning.
Extinction learning is attenuated in (A) humans with the BNDF Met (M) allele relative to non-Met allele (V) as measured by change in galvanic skin response **P<0.01. (B) This finding is paralleled in the BDNF SNP knock-in mice measured by less change in freezing behavior with repeated presentation of the conditioned stimulus alone during extinction trials *P<0.05 (VV vs. VM/MM). Adapted with permission from (Soliman et al., 2010). Extinction learning is enhanced in (C) humans with the FAAH A allele relative to C allele as measured by greater change in galvanic skin response. *P<0.05 (D) This finding is paralleled in the FAAH SNP knock-in mice measured by decreased freezing behavior with repeated presentation of the conditioned stimulus alone during extinction trials. **P < 0.01 homozygous knock-in mice vs wild-type controls; P<0.05 heterozygotes vs wild type. Adapted with permission from (Dincheva et al., 2015).
Frontoamygdala circuitry
As with the behavioral analysis of fear extinction learning, we generated a priori hypotheses based on understanding of the neurocircuitry underlying fear responses and the observed extinction learning phenotypes. We then implemented a variety of species-specific techniques to assess functional and structural connectivity in the frontoamygdala circuit as a function of genotype. Specifically, we hypothesized that impaired extinction learning related to the BDNF Met allele would be accompanied by reduced connectivity from prefrontal cortex and amygdala. Conversely, we hypothesized that the FAAH A allele would be associated with enhanced frontoamygdala connectivity.
In human subjects who were scanned with fMRI during fear extinction learning the BDNF Met allele was associated with decreased activation of the vmPFC and increased amygdala activation consistent with the behavioral effects of the BDNF Met allele. Structural connectivity in the frontoamygdala circuit was assessed by diffusion tensor imaging (DTI). The BDNF Met allele was associated with reduced fractional anisotropy reflecting white matter density in the uncinate fasiculus connecting vmPFC and amygdala. In mice extinction induced activation of vmPFC was assessed by c-Fos expression which also showed hypoactivation of vmPFC neurons in response to extinction learning in BDNF Met allele mice. Electrophysiological analysis of spike timing induced plasticity in the infralimbic cortex of wild type and BDNF Met mice identified reduced enhancement of post-synaptic responses involving both N-methyl-D-aspartate and gamma-aminobutyric acid receptors (Pattwell et al., 2012a).
We also tested structural and functional effects of the FAAH C385A polymorphism on frontoamygdala circuitry. In humans, we examined resting state connectivity between the subgenual vmPFC and amygdala and the dorsal ACC and amygdala. FAAH A allele carriers showed region-specific increases in correlated blood oxygen level-dependent (BOLD) signals in the vmPFC and the bilateral amygdala. In mice invasive tract tracing allowed a more detailed assessment of the neuroanatomical location and directionality of genotypic differences in frontoamygdala circuitry (Dincheva et al., 2015). We injected anterograde and retrograde tracers into the IL and quantified tracer transport to basolateral amygdala. The FAAH A allele induced a selective increase in anterograde tracer density of IL to BLA but no effect on BLA to IL tracer density. This selective increase in descending IL-amygdala projections provides a neuroanatomical basis for the increased functional connectivity in frontoamygdala circuitry in human A allele carriers and may help explain reported genotypic differences in fear regulation.
Gene x Development Interactions
In addition to identifying effects of BDNF Val66Met and FAAH C385A on fear extinction learning and the supporting frontoamygdala circuit, the cross species studies demonstrated that knock-in mice with common human variant alleles recapitulate human phenotypes at complex levels of biology and behavior. This validation opens the door to using polymorphic knock-in mice in exploratory analyses to identify the detailed effects of common polymorphisms across behavioral domains and developmental stages ultimately to develop genetic biomarkers to enhance developmentally informed interventions and treatment selections. Exploring gene x development interactions in mice offers many advantages over human studies including homogeneous genetic background, controlled breeding of known genotypes, ability to control environmental exposures across development, and invasive analyses. The ability to perform controlled experiments on human polymorphisms in a validated model system should improve the reliability of human behavioral association studies to the point where they can be more effectively translated to clinical practice (Casey et al., 2014; Glatt and Lee, 2015).
We recently used the BDNF Val66Met mice to extend our earlier studies on adolescent suppression of contextual fear expression (Pattwell et al., 2011). In those studies we found that hippocampaldependent contextual fear associations can be efficiently acquired during adolescence, but not retrieved until adulthood. Previously we have shown that adult BDNF Met mice have a deficit in acquisition of contextual fear associations perhaps related to high BDNF dependence of hippocampal plasticity reflected in its high level of BDNF expression. As a result, BDNF Met mice that undergo contextual fear learning in adolescence do not display fear responses to the context in adulthood while wild type mice do (Dincheva et al., 2014). This finding demonstrates the complexity of interactions between molecular function, development, and behavioral sub-domains and that a polymorphism may confer both risk and resilience for anxiety depending on the timing and nature of fear exposures.
Detailed assessment of the BDNF Val66Met and FAAH C385A mice across the lifespan will determine how these polymorphisms alter the developmental course of fear learning. These effects may be to shift developmental trajectories to older or younger ages, expand particular developmental stages, or to alter expand or close sensitive periods of development. All of these effects can influence risk for and therapeutic opportunities to treat anxiety disorders. Ultimately, exploratory analyses in polymorphic mice can generate and refine hypotheses for association testing in human population samples.
Targeting Treatments by Age and Genetics
The developmental changes we have described in fear learning behaviors and their underlying neurocircuitry help to explain the discrete peak in incidence for anxiety disorders in adolescence. Individual genetic variation can alter normative development and interact with development to alter the risk for or timing of anxiety. Age and genetics can also affect how well an individual may respond to treatments that target these fear learning behaviors. As a result, an appreciation for the developmental stage and genetic composition can enhance targeting treatments to individuals with the greatest capacity to benefit from them enhancing clinical outcomes.
The Child/Adolescent Anxiety Multimodal Study (CAMS) study compared treatments in individuals aged 7-17 with diagnoses of separation anxiety, generalized anxiety disorder or social phobia (Walkup et al., 2008). Across this age range they found that cognitive behavioral therapy (CBT) consisting of fourteen sessions of training in anxiety-management skills, followed by behavioral exposure to anxiety-provoking situations performed similarly to pharmacotherapy, consisting of the SSRI sertraline, both of which performed better than placebo. Combination sertraline and CBT therapy was associated with higher response rates than either treatment alone which may have biological significance beyond simply ‘more is better’ because SSRI’s have been shown to enhance behavioral and neural plasticity, including retention of fear extinction and can even re-establish visual plasticity in adulthood outside of the normal visual critical period (Hensch and Bilimoria, 2012; Karpova et al., 2011).
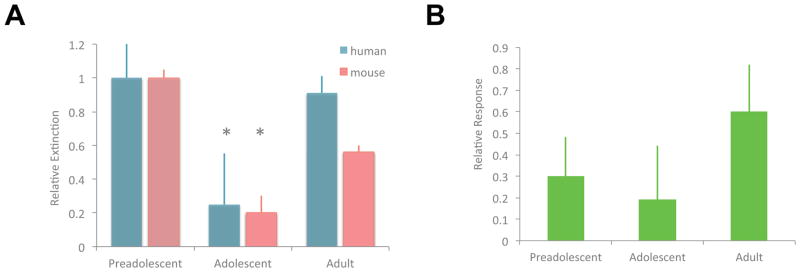
Motivated by the hypothesis that diminished fear extinction in adolescence would hinder response to an exposure-focused CBT relative to children and adults, we analyzed these clinical trial data to compare treatment responses in three developmental groups and confirmed that adolescent age group displayed nonsignificant lower rates of treatment response than children and adults (Drysdale et al., 2014)(Figure 2B). This result suggests that anxiety in adolescents may be better treated through modalities that do not depend upon fear extinction learning, or may require more intensive extinction-based treatments, and/or may require combination therapy with an SSRI to enhance the typically inefficient extinction processes in adolescence.
Individual genetic factors can also affect response of anxiety disorders to behavioral therapies. Our previous report of cross-species effects of the BDNF Val66Met polymorphism on fear extinction learning, led Felmingham and colleagues to genotype subjects with PTSD treated with exposure-based therapy. They found, as predicted, that BDNF Met allele carriers responded less well than Val allele homozygotes (Felmingham et al 2012). This finding suggests that genotyping patients with PTSD and perhaps other anxiety disorders may inform treatment decisions. Carriers of the BDNF Met allele may require more intensive exposure-based therapies or might be more amenable to validated therapies that emphasize normalization of interpersonal relationships and presumably work through distinct neural mechanisms (Markowitz et al., 2015).
Finally, recent studies have shown an alternative method for attenuation of fear memories beyond basic fear extinction learning-that of memory reconsolidation update (Monfils et al., 2009; Schiller et al., 2010). Memory reconsolidation is based on the principle that memories are dynamic rather than stable and every time a memory is retrieved, it returns to a fragile state and must be restabilized or become diminished. Recent human imaging studies suggest that reconsolidation of fear memory is primarily mediated by the amygdala rather than on prefrontal circuitry (Agren et al., 2012; Schiller et al., 2013). These findings suggest a plausible way in which adolescents may be able to overcome pathologic fear memories via interventions that alter memories within the amygdala such as reconsolidation update. We recently compared the retention of a fear memory after extinction learning with and without a preceding reminder cue in both adolescents and adults (Johnson and Casey, 2015). We found that even though adolescents displayed reduced fear extinction relative to adults, those who were required to retrieve the fear association prior to extinction learning (i.e. reconsolidation) had a dramatically reduced fear memory the next day compared to extinction only. In fact, adolescent fear memories were diminished to the same degree as they were in adults (Figure 6). While extinction learning involves the encoding of a new competing memory that leaves the original fear memory intact, the current results suggest that the safety information provided during post-retrieval extinction (reconsolidation update) is integrated into the original fear memory altering its affective value even in the absence of fear extinction learning. Thus, incorporating memory reconsolidation update into exposure therapies for anxiety may improve outcomes for adolescents.
Figure 6. Leveraging behavioral sub-domains to overcome developmental liabilities.
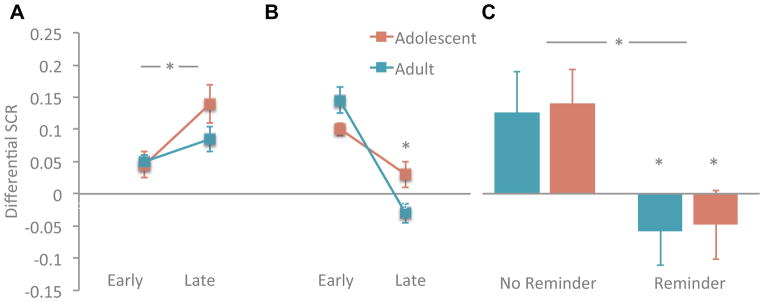
In adolescence acquisition of cued-fear associations is intact (A) relative to adults (*P<0.05 early vs late trials) but (B) extinction (difference in early vs late extinction trials reduced *P<0.05) contributing to risk for anxiety disorders and response to treatment. (C) Reconsolidation of fear associations is intact in adolescents and can reduce fear memories as well as in adults (difference in fear response with vs without reminder cue P<0.05) between suggesting that adding reconsolidation to exposure-based therapy for adolescents would improve clinical response. Adapted with permission from (Johnson and Casey, 2015).
Implication/Future Directions
We are at a point in time of both tremendous opportunity and obligation to advance our understanding of how to treat the developing brain (Lee et al., 2014). By understanding sensitive windows of development when the brain is especially receptive to the environment, we may be able to understand shifts and early closures of these windows due to environmental or genetic factors and potentially expand them behaviorally and/or pharmacologically. Moreover we could shift our focus from targeting immature brain systems based on adult human and animal research toward more developed or dynamic/plastic circuitry to enhance the efficacy of our treatments. These efforts to guide novel interventions will require bridging across humans and animal model systems at the genetic, molecular, circuit, and behavioral levels. This approach will allow for more precision in targeting treatments by the age and genetic makeup of the individual and together with policies for modifying the environment will ultimately diminish the high psychological and economic toll of mental illness on young people, their families and society.
Acknowledgments
This work was supported, in part, by the National Institute of Mental Health P50 MH079513 (BJC, CEG, FSL); a generous gift by the Dr. Mortimer D. Sackler family; Dewitt-Wallace Reader’s Digest Fund, Citigroup Biomedical Imaging Center and Imaging Core, Pritzker Neuropsychiatric Disorders Research Consortium, and the NewYork Presbyterian Youth Anxiety Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agren T, Engman J, Frick A, Bjorkstrand J, Larsson EM, Furmark T, Fredrikson M. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Cravatt BF. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert opinion on drug discovery. 2009;4:763–784. doi: 10.1517/17460440903018857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learning & memory. 2012;19:598–604. doi: 10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes, brain, and behavior. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends in neurosciences. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral neuroscience. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental psychobiology. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychol Rev. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental review : DR. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Altemus M, Pattwell S, Jones R, Levita L, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Pattwell SS, Glatt CE, Lee FS. Treating the developing brain: implications from human imaging and mouse genetics. Annual review of medicine. 2013;64:427–439. doi: 10.1146/annurev-med-052611-130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science’s STKE : signal transduction knowledge environment. 2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learning & memory. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. The Journal of comparative neurology. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Pattwell SS, Tessarollo L, Bath KG, Lee FS. BDNF modulates contextual fear learning during adolescence. Developmental neuroscience. 2014;36:269–276. doi: 10.1159/000358824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Hartley CA, Pattwell SS, Ruberry EJ, Somerville LH, Compton SN, Lee FS, Casey BJ, Walkup JT. Fear and anxiety from principle to practice: implications for when to treat youth with anxiety disorders. Biol Psychiatry. 2014;75:e19–20. doi: 10.1016/j.biopsych.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of general psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA. The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry. 2013;73:1059–1063. doi: 10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt CE, Lee FS. Common Polymorphisms in the Age of Research Domain Criteria (RDoC): Integration and Translation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual review of neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Bilimoria PM. Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum : the Dana forum on brain science. 2012;2012:11. [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learning & memory. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Casey BJ. Extinction during memory reconsolidation blocks recovery of fear in adolescents. Sci Rep. 2015;5:8863. doi: 10.1038/srep08863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, Antila H, Popova D, Akamine Y, Bahi A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Wakako R, Komori T, Shigemi H, Miyazaki N, Ito H, Kumagai T, Tsuzuki M, Shigemi K, Yoshida F, et al. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2007;25:367–372. doi: 10.1016/j.ijdevneu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual review of public health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical Analyses of Long-Term Extinction of Conditioned Fear in Adolescent Rats. Cerebral cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behavioral neuroscience. 2007;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning & memory. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of cognitive neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. Journal of neurochemistry. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental neuroscience. 2012;34:101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, Casey BJ. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Gorzalka BB. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. 2012;204:17–30. doi: 10.1016/j.neuroscience.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Lee TT, Hill MN, Hillard CJ, Gorzalka BB. Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse. 2013;67:4–10. doi: 10.1002/syn.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and biobehavioral reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Markowitz JC, Petkova E, Neria Y, Van Meter PE, Zhao Y, Hembree E, Lovell K, Biyanova T, Marshall RD. Is Exposure Necessary? A Randomized Clinical Trial of Interpersonal Psychotherapy for PTSD. The American journal of psychiatry. 2015 doi: 10.1176/appi.ajp.2014.14070908. appiajp201414070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EM, Morishita H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Frontiers in psychiatry. 2013;4:146. doi: 10.3389/fpsyt.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012a;32:2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, et al. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences of the United States of America. 2012b;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20:10371046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal--prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, Leibenluft E, Pine DS. Fear Conditioning and Extinction in Anxious and Nonanxious Youth and Adults: Examining a Novel Developmentally Appropriate Fear-Conditioning Task. Depression and anxiety. 2014 doi: 10.1002/da.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current opinion in neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current opinion in neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain research. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. The New England journal of medicine. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CS, Richardson R. Extinction in the developing rat: an examination of renewal effects. Developmental psychobiology. 2007;49:565–575. doi: 10.1002/dev.20244. [DOI] [PubMed] [Google Scholar]