Figure 6.
Clearance of Stress-Induced Protein Aggregates Is Severely Compromised in Yeast with Unmodified U34
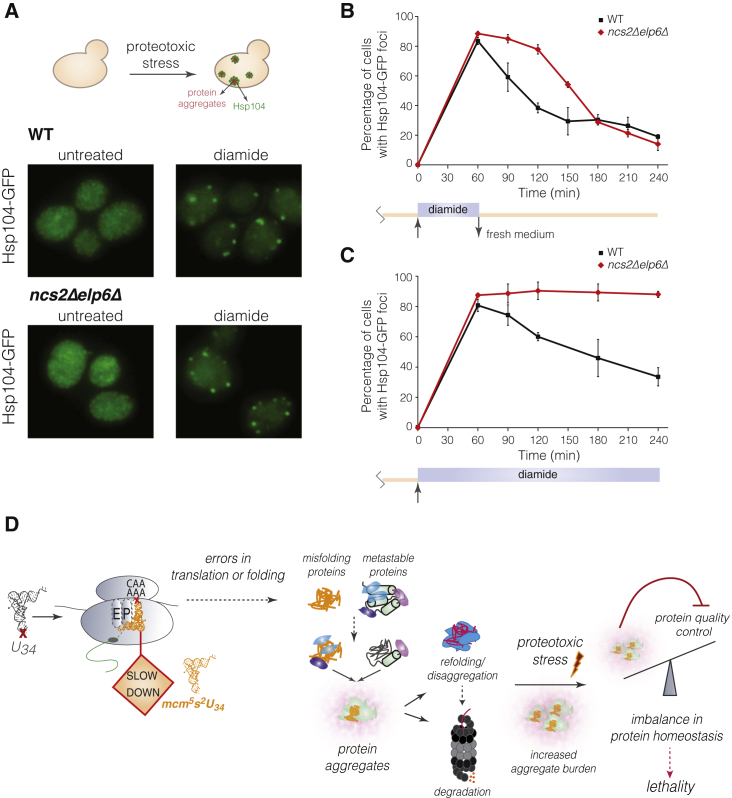
(A) Intracellular distribution of Hsp104-GFP in WT and ncs2Δelp6Δ yeast grown in YPD (left) or treated with 1.5 mM diamide for 60 min (right).
(B) Percentage of WT (black squares) and ncs2Δelp6Δ cells (red diamonds) with Hsp104-GFP-containing aggregates upon transient exposure to diamide. Exponentially growing cells were treated with 1.5 mM diamide for 60 min and returned to fresh YPD medium. At least 100 cells from three different fields were counted per time point (mean ± SD; n = 3).
(C) Percentage of WT and ncs2Δelp6Δ cells with Hsp104-GFP-containing aggregates in the continuous presence of 1.5 mM diamide. Quantification was performed as in (B).
(D) Model for the molecular consequences of aberrant U34 modification.