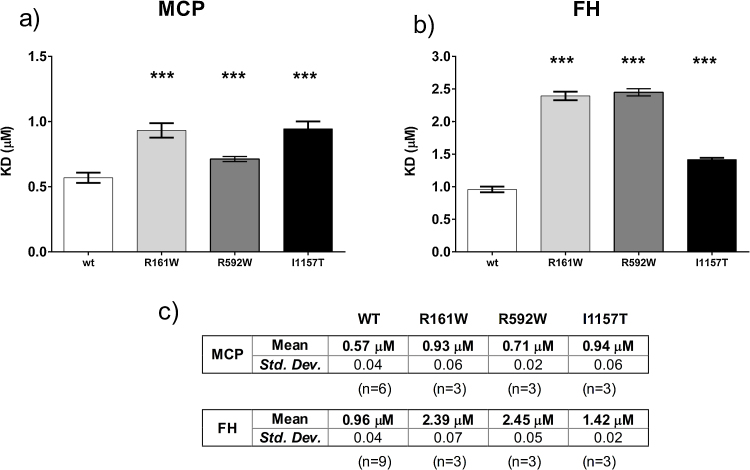
Fig. 4.
Affinity assays of MCP and FH for the mutant C3 mutants. Binding affinities of C3b mutants with complement regulators MCP and FH were measured by surface plasmon resonance (SRP). (a) Recombinant sMCP binding affinities (KD, μM) with C3bR592W (0.71 ± 0.02 μM; P = 0.0006, n = 3), C3bR161W (0.93 ± 0.06; P < 0.0001, n = 3), and C3bI1157T (0.94 ± 0.06 μM; P < 0.0001, n = 3); all showing weaker affinity compared to wt C3b (KD 0.57 ± 0.04 μM, n = 6). (b) FH binding affinities (KD, μM) with C3bR592W (2.45 ± 0.05 μM; P < 0.0001, n = 3), C3bR161W (2.39 ± 0.07; P < 0.0001, n = 3), and C3bI1157T (1.42 ± 0.02 μM; P < 0.0001, n = 3); all showing weaker affinity compared to wt C3b (KD 0.96 ± 0.04 μM, n = 9). P-values (two-tailed, unpaired t-test; depicted as asterisk) refer to the comparison of each mutant KD versus wt KD. (c) Table summarizing affinity (KD, μM; Mean ± standard deviation) values of recombinant sMCP or FH binding to immobilized wt and mt C3b.