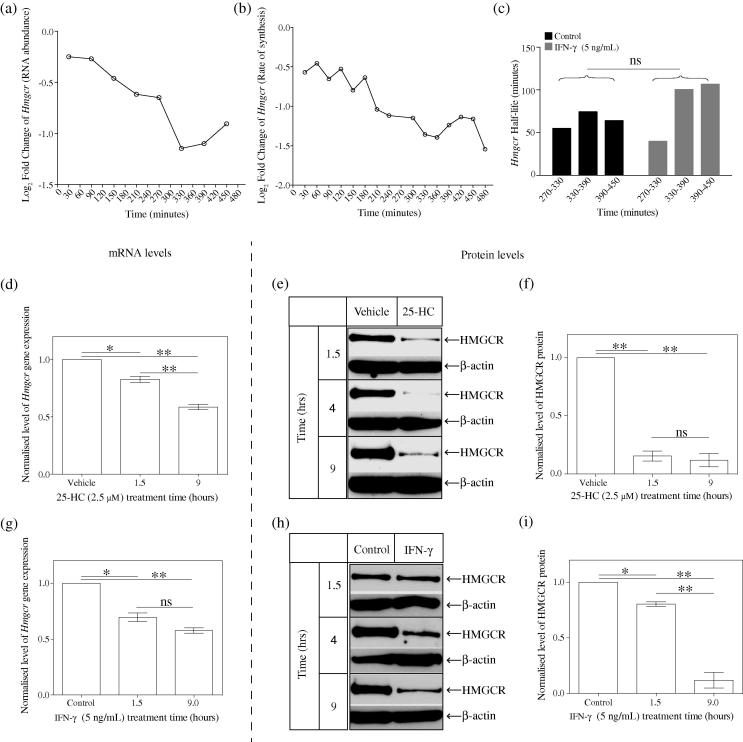
Fig. 3.
Time-course showing the Hmgcr mRNA and HMGCR protein levels following IFN- treatment. (a) Temporal alterations in abundance of Hmgcr transcript in IFN- treated BMDMs (relative to control treated) in Medium A over an 8-h period. Each point represents overall abundance of transcript at the indicated time. Relative log fold change values were calculated by subtracting the control treated from the IFN--treated signal values. p = 2.72E−05. (b) Temporal alterations in de novo synthesis rate of Hmgcr transcript in IFN--treated BMDMs (relative to control treated) in Medium A over an 8-h period. Each point represents transcript synthesis rate in control treated vs IFN- during a 30-min period. Normalised log fold change values were calculated by subtracting the control treated from the IFN--treated signal values. p = 2.93E−11. (c) Half-life values of Hmgcr at indicated period time. Based on the RNA abundance and newly transcribed RNA levels of Hmgcr, the half-lives of Hmgcr for each period were calculated as previously described [39]. (d) Wild-type BMDMs were treated with 2.5 μM of 25-HC in Medium C at multiple time points and Hmgcr mRNA level was determined by qRT-PCR. Data are mean ± SEM (n = 3). (e) Western blot analysis of the HMGCR protein level with 2.5 μM of 25-HC treatment in Medium C at multiple time points. (f) Intensity values of HMGCR to -actin. Data are mean ± SEM (n = 3). (g) Wild-type BMDMs were treated with 5 ng/mL of IFN- in Medium C at multiple time points and Hmgcr mRNA level was determined by qRT-PCR. Data are mean ± SEM (n = 3). (h) Western blot analysis of HMGCR protein level with 5 ng/mL of IFN- treatment in Medium C at multiple time points. (i) Intensity values of HMGCR to -actin. Data are mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, determined with an unpaired Student’s t test.