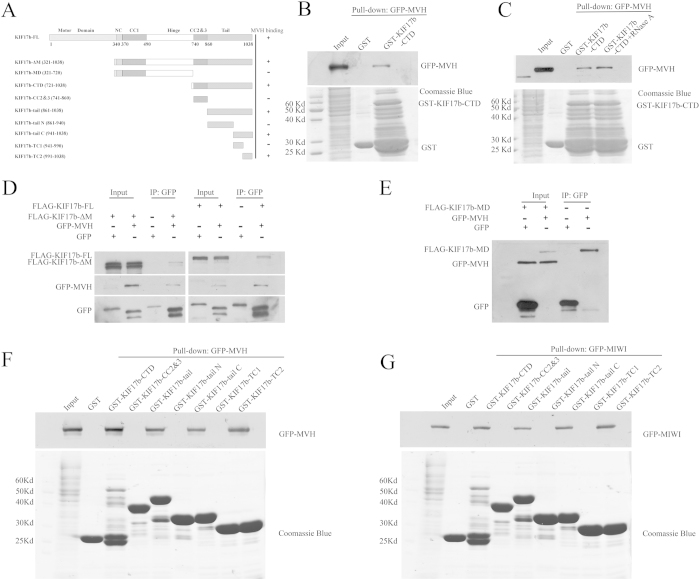
Figure 4. Interaction of MVH and MIWI with the tail of KIF17b.
(A) Schematic diagram of the full length and truncations of KIF17b; the residue numbers at the domain boundaries are indicated. (B,C) The interaction of KIF17b-CTD with MVH was assayed by the GST pull-down assay. Purified GST-KIF17b was used to absorb GFP-MVH from the lysates of HEK293T cells. The bound materials were then subjected to SDS-PAGE and immunoblotted with anti-GFP antibody or treated with RNase A before SDS-PAGE. The GST-fusion proteins were shown by Coomassie brilliant blue R-250 staining of the gels. (D) Immunoprecipitation of KIF17b by MVH. Expression plasmid of the full length of FLAG-tagged KIF17b, or plasmid of the motor-domain-deleted form of FLAG-tagged KIF17b (FLAG-KIF17b-ΔM) and GFP-tagged MVH were co-transfected to HEK293T cells, and the cell lysates were precipitated by the anti-rabbit GFP antibody, and immunoblotted with anti-mouse GFP antibody or anti-mouse FLAG antibody. (E) Plasmids of the middle domain of KIF17b (FLAG-KIF17b-MD) and GFP-MVH were transfected into HEK293T cells and then the cell lysates were immunoprecipitated by the GFP antibody. (F,G) Mapping of the MVH and MIWI binding sites of KIF17b-CTD was completed using the GST pull-down assay. Purified truncations of GST-KIF17b-CTD were used to precipitate GFP-MVH or GFP-MIWI from the lysates of HEK293T cells. The precipitates were then subjected to SDS-PAGE and immunoblotted with anti-GFP antibody; the gels were then stained by Coomassie brilliant blue R-250.