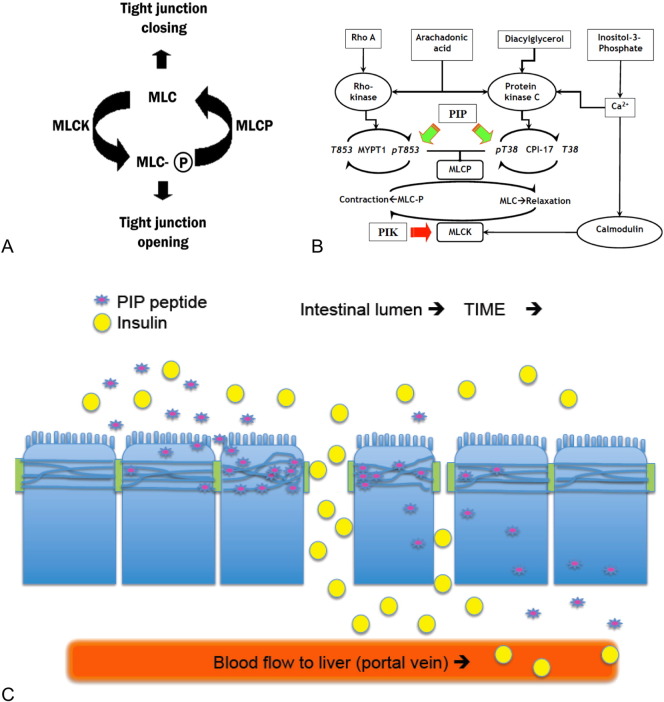
Fig. 7.
Conceptual aspects of the PIP peptides strategy. (A) General relationship between TJ barrier function and MLC phosphorylation status. (B) Schematic representation of mechanisms regulating the activity of MLC kinase and MLC phosphatase. Ca2 +/calmodulin-mediated activation of MLC kinase (MLCK) causes contraction of the actomyosin cytoskeleton associated with TJ structures, resulting in dilation of the space between adjacent epithelial cells–enhanced paracellular permeability. The PIP (permeant inhibitor of phosphatase) peptides (green arrows) described in these studies were designed to disrupt MYPT1 or CPI-17 regulation of MLCP function. PIK (permeant inhibitor of kinase) action on MLCK is noted by a red arrow. (C) Cartoon depicting several of the dynamic factors affecting the PIP peptide-mediated enhancement of insulin uptake. PIP peptide (purple star) entry into epithelial cell and modulation of MLCP function at the TJ to open the paracellular route to solutes, a transient effect due to the systemic uptake, dilution, and elimination. Co-administration of insulin (yellow circles) with a PIP peptide facilitates uptake of this hormone into the portal vein. The location and duration of insulin uptake is dependent upon sufficient adjacent PIP concentration and actions. Movement along the intestinal lumen over time (arrows) and dilution into the luminal contents should affect both PIP peptide actions and the extent of insulin uptake.