Figure 7.
Summary of Peptide Recognition and Selectivity Determinants for CLR:RAMP1-3 Complexes
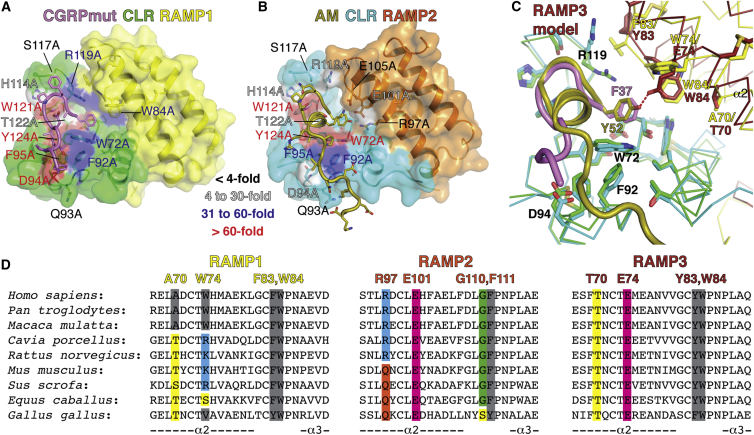
(A and B) Structures of the CGRPmut- and AM-bound CLR:RAMP1/2 ECD heterodimers with the surface of receptor residues colored according to their effect on CGRP (A) or AM (B) signaling potency when mutated to alanine. Color coding signifies the extent of reduced signaling potency at intact receptor complexes in cells compared to wild-type as indicated by the inset legend. RAMP1 W84A data are from Moore et al. (2010) (34-fold reduced potency).
(C) Model for RAMP3 binding site augmentation. A homology model of the AM-bound CLR:RAMP3 ECD complex (Supplemental Experimental Procedures) was superimposed with the CGRPmut-bound CLR:RAMP1 and AM-bound CLR:RAMP2 ECD structures based on the CLR positions. Only the RAMP3 subunit from the homology model is shown and RAMP2 is omitted. The receptors are shown as Cα traces and selected residues as sticks.
(D) Amino acid sequence alignments for RAMP1-3 from the indicated species showing the α2 and α2-α3 loop regions that augment the peptide-binding site.