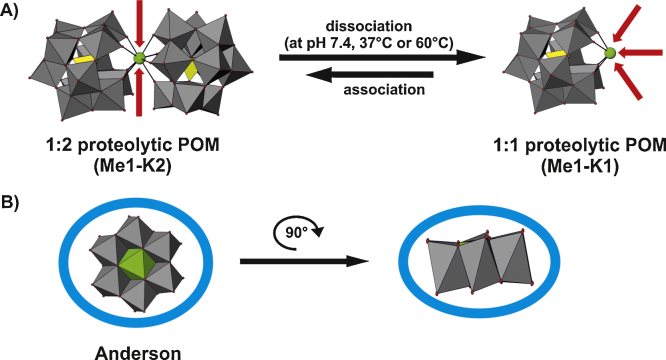
Fig. 6.
Structural comparison between a hydrolytically active POM and the well-known, proteolytically passive Anderson POM [59]. (A) The proteolytic POM (Me1-K2; Me = metal, K = Keggin) consists of one hydrolytically active metal (shown as a green sphere, Me1) and two Keggin structures (addenda atoms are depicted as gray polyhedra, oxygen atoms as small red spheres and the incorporated heteroatoms as yellow polyhedra, K2), which is likely to dissociate into the monomeric 1:1 species (Me1-K1) at pH 7.4 and 37 °C (for the [Ce(α-PW11O39)2]10−) or 60 °C (for the Zr(IV)–POM complexes), respectively. Red arrows indicate the accessibility of the active metal, which is increased after dissociation. (B) The incorporated metal atom (depicted as a green polyhedron) is shielded by the POM scaffold (addenda atoms are illustrated as gray polyhedra and oxygen atoms as small red spheres) which is indicated by blue circles around the POM. The Anderson POM is rotated around 90° to show it from another perspective (side view) which clearly shows the inaccessibility of the metal. Note that the incorporated heteroatom (yellow polyhedron) of the Keggin structure in (A) is also shielded, so that no direct interaction of this atom with proteins is observed.