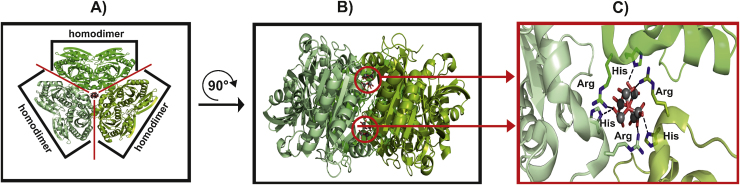
Fig. 13.
Structure of Uridine Phosphorylase from Escherichia coli. (A) The protein is a homohexamer of which structure can be described as the assembly of three homodimers. The protein is shown as cartoon, each homodimer colored in a different shade of green. The [V7O19]3− anions are shown as ball and stick (color code: vanadium atoms = gray, oxygen atoms = red) but only 6 VOx units were modeled in the structure, because the authors used the trimeric head of the metavanadate from PDB entry 1DKT as a model [96]. (B) Side view of (A) to better demonstrate the presence of the two [V7O19]3− which are lying above each other along the threefold-axis. (C) Illustration of the [V7O19]3−–protein interaction, where the interacting residues are depicted as ball and stick (color code: carbon atoms = green, nitrogen atoms = blue). Every interacting monomer is contributing one nitrogen from an arginine and one nitrogen from a histidine to the electrostatic interaction with each [V7O19]3− molecule.