Fig. 15.
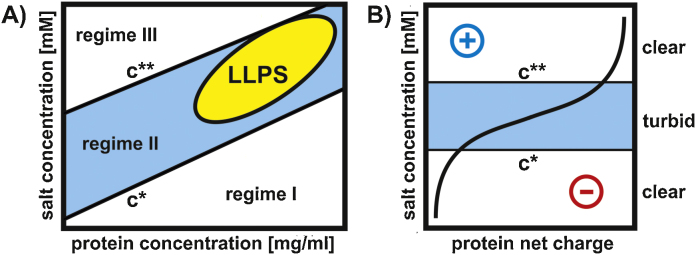
Phase behavior of a solution containing an acidic protein (e.g. human serum albumin) in the presence of a multivalent cation (e.g. Y3+). (A) The phase diagram demonstrates that the reentrant condensation phase behavior consists of three regimes (regime I, II and II) which are separated by two critical salt concentrations, c* and c**, where c* indicates the salt concentration at which the protein solution becomes turbid with increasing salt concentration and c** the salt concentration at which the solution becomes clear again upon further increase of the salt concentration. Regime II (in between c* and c**) contains a phase separation region, the so-called liquid–liquid-phase separation region (LLPS). (B) The charge inversion of the protein is shown as a function of salt concentration. The charge inversion takes place within regime II, where the solution is turbid because the surface of the acidic protein is gradually saturated by the multivalent cations until the surface charge is completely neutralized and the protein reaches its lowest solubility (during the course the LLPS region is traversed if the protein concentration is high enough). Further increase of the salt concentration (>c**) leads to the attachment of more cations giving the protein a positive net charge which increases the solubility of the protein again and making the protein solution clear again. This figure is a modified version from reference [122], which was kindly provided by Schreiber, Tübingen, Germany.
