Abstract
BACKGROUND AND PURPOSE
Intramedullary spinal cord neoplasms (ISCN) in children provide diagnostic, treatment and management dilemmas. Resection results in the best chance for disease control, but the greatest risk of neurologic deficit. We hypothesize that diffusion tensor imaging (DTI) and diffusion tensor-fiber tracking (DT-FT) can help characterize margins of pediatric ISCN to aid in surgical planning.
METHODS
This HIPAA compliant retrospective study was performed after Institutional Review Board approval. Patients with ISCN from a single tertiary care pediatric institution were identified, and patients with preoperative DTI were evaluated.
RESULTS
Ten patients (8 males, 2 females) with ISCN with preoperative DTI were identified. The mean age was 11.1 ± 6.2 years (range 2 – 18 years). Eight tumors demonstrated DTI and DT-FT evidence of splayed cord tracts, and two demonstrated evidence of infiltration of cord tracts. The eight patients with splayed tracts underwent resection, with seven achieving gross-total resection and one subtotal resection. The two patients with infiltration of white matter tracts underwent biopsy of their lesion.
DISCUSSION
DTI of pediatric ISCN can aid in defining the margins of the tumor and relationship to the intrinsic white matter structures of the spinal cord. Splaying and displacement of fiber tracts appears to predict a discrete margin to the tumor and resectability, while infiltration of the white matter tracts suggests biopsy may be more advisable.
INTRODUCTION
Intramedullary spinal cord neoplasms (ISCN) represent up to 6% of pediatric CNS neoplasms [1] and pose diagnostic and treatment dilemmas. Surgical resection is most effective at reducing disease burden, but carries significant risks of morbidity. Differentiating discretely marginated from infiltrating tumors is important for surgical planning, although complicated by peritumoral edema and the often heterogeneous nature of these lesions.
Advances in magnetic resonance imaging (MRI) technology have allowed preoperative characterization of brain tumors to include physiologic parameters in addition to traditional structural evaluation [2]. Diffusion tensor imaging (DTI) and diffusion tensor-fiber tracking (DT-FT, i.e. “tractography”) are noninvasive methods to estimate the direction and integrity of white matter tracts. While this technique has traditionally been employed in the brain, recent investigations have evaluated its role in the spinal cord in the setting of degenerative changes, trauma, and neoplasm [3-6]. Early reports in adults have suggested that DTI and DT-FT can aid in differentiating astrocytomas from ependymomas, because of the typical infiltrating nature of astrocytomas as compared to ependymomas, which tend to displace rather than disrupt white matter tracts [7-9]. Because of the difficulty in translating DTI to the spine, and the rarity of ISCN, the true role of this technique has not yet been established.
There have been no reported case series correlating DTI with the histologic characterization of ISCN in children. We hypothesized that DTI and DT-FT could aid in determining resectability; however, it may be a less reliable means of differentiating ependymomas from astrocytomas compared to adults. Spinal cord astrocytomas in children often have different histologic characteristics than those seen in adults. We evaluated whether DTI and DT-FT can be used to characterize the margins of intramedullary spinal cord neoplasms in children by assessing the infiltrative or discrete nature of the lesions and their anatomic relationship with surrounding white matter tracts.
METHODS
Patient Selection and Neurologic Assessment
This HIPAA compliant retrospective study was performed after Institutional Review Board approval. Patients with ISCN and preoperative DTI from a single tertiary care pediatric institution were identified. Cases from 2011 through 2013 were evaluated, as spinal cord DTI was started in 2011. Neurologic exams were performed on each patient preoperatively, at the time of discharge, and at follow-up.
Magnetic Resonance Imaging
Studies were performed on a 3-tesla MRI scanner (Siemens Verio, Siemens, Munich, Germany) using 20 directions of encoding, and on a GE 1.5 or 3.0 Tesla (Signa HDxt, General Electric, Milwaukee, WI) scanner with 25 directions of encoding. All DTI data was acquired in both the axial and sagittal plane with 2 mm slice interval and a 128 × 128 matrix, b value of 1000 msec with a single b0 value, TE of 86msec and TR of 10,000msec. Axial DTI was acquired to include at least 2 cm of uninvolved cord along both the superior and inferior margins of the lesion, as determined by the neuroradiologist evaluation of conventional structural imaging at the time of acquisition. At the time of DTI acquisition, conventional MRI images of the spine were obtained prior to and after gadolinium administration.
Vendor-provided software was used for distortion correction and processing of fractional anisotropy (FA), directionally encoded fractional anisotropy maps (DE-FA), and DT-FT overlays. DT-FT was also evaluated using one of two dedicated workstations (StealthVis, Medtronic, Inc, Memphis, TN; Dynasuite Neuro 3.0, InVivo Corp, Pewaukee, WI). DT-FT was processed using a fiber assignment by continuous tracking algorithm [10] with an FA threshold of 0.2, with ROI placed by the neuroradiologist supervising the study. ROI placement was performed on multiple axial images extending from approximately 2 cm above the lesion to approximately 2 cm below the lesion, at no greater than 1 cm intervals. At each level evaluated, an ROI for DT-FT seeding was placed involving the entire cross-section of the cord and ROIs covering each of four quadrants demarcated by a midline anterior and posterior axis. Additional ROIs were placed using neuroradiologist discretion to help further scrutinize tumor margins, as needed.
Evaluation of Imaging
All spinal cord tumors were assessed by MRI for size, signal characteristics, solid and/or cystic nature, degree of enhancement and location. All MRI images were interpreted by a board-certified fellowship-trained neuroradiologist who performed DTI and DT-FT processing. With each case, a preoperative consultation was performed with a pediatric neurosurgeon prior to resection or biopsy. Retrospective review of the structural and diffusion tensor data was performed by consensus of two board-certified neuroradiologists.
Surgical Treatment
Based upon the clinical presentation and the imaging appearance, the surgical plan was either a biopsy or resection. When the plan was for resection, the goal was to maximally resect tumor while preserving the fibers (and function) of the native spinal cord. All resections were performed with laminectomy or laminoplasty, dorsal midline myelotomy, and microsurgical resection. Intraoperative monitoring with sensory evoked potentials and motor evoked potentials was used in all cases with intended resection. Surgical records were evaluated to determine description of the characteristics of the tumor as well as the tumor-cord interface. Post-operative notes, including discharge summaries and clinic followup notes were evaluated to determine if there were new or deteriorating neurologic symptoms.
RESULTS
Ten patients with ISCN with preoperative DTI were identified, including eight males and two females. The mean age was 11.1 ± 6.2 years (range 2 – 18 years, median 13 years). There were three cervicomedullary junction tumors, four cervical tumors and three thoracic tumors. The histologic diagnoses were pilocytic astrocytoma (n = 7), ependymoma (n = 1), ganglioglioma (n = 1), and high grade glioma (n = 1; Table 1). The patient with the ependymoma had undergone previous subtotal resection of the lesion at a separate institution.
Table 1.
| Age | Gender | Presentation | Location | MRI appearance | DTI | Surgical Margins | Result | Histology |
|---|---|---|---|---|---|---|---|---|
| 4 | M | Quadriparesis, sig R hemiparesis | Cervicomedullary | Solid enhancing lesion with cystic areas | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 5 | M | Back pain | Thoracic | Cystic lesion with solid enhancing (Figure 1) | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 12 | M | CT | Cervicothoracic (C3-T2) | Solid enhancing lesion with cystic areas | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 17 | F | Gait disturbances | Thoracic | Mixed solid and cystic with internal areas of hemorrhage and dysplastic blood vessels, extensive thoracic syrinx, | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 18 | M | Upper extremity weakness | Cervical | Solid enhancing lesion with cystic area, fluid level from prior hemorrhage | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 6 | M | Progressive hemiparesis | Cervicomedullary | Solid enhancing lesion with cystic areas | Splayed (non-infiltrated) | GTR | Discrete | JPA |
| 17 | M | Progressive weakness, hx of prior ependymoma resection | Cervical | Heterogeneous mass with solid and cystic areas, evidence of prior hemorrhage | Splayed (non-infiltrated) | GTR | Discrete | Ependymoma |
| 2 | M | Gait disturbances | Cervicomedullary | Solid enhancing lesion with cystic areas (Figure 2) | Splayed (non-infiltrated) | STR | Discrete | JPA |
| 14 | M | Left jaw pain | Cervical | Enhancing nodular region with surrounding non-enhancing T2 signal abnormality (Figure 3) | Disrupted | Biopsy | Ill-defined | Ganglioglioma |
| 16 | F | Lower extremity weakness | Thoracic | Peripherally enhancing irregular lesion, extensive leptomeningeal disease | Disrupted | Biopsy | Ill-defined | High grade glioma |
CM, cervicomedullary; CT, cervicothoracic; C, cervical; T, thoracic; GTR, gross total resection; JPA, juvenile pilocytic astrocytoma; STR, subtotal tumor resection
Eight tumors showed evidence of splaying of fibers, including all seven pilocytic astrocytomas and the ependymoma (Figure 1). Subtotal resection was planned on one large cervicomedullary junction pilocytic astrocytoma (Figure 2), and gross-total resection was planned on the remaining tumors that showed splaying of fibers. In patients undergoing resection, no new neurologic deficits persisted beyond hospital discharge. The ganglioglioma and high-grade glioma showed evidence of infiltration of fibers (Figure 3), and biopsy was performed.
Figure 1.
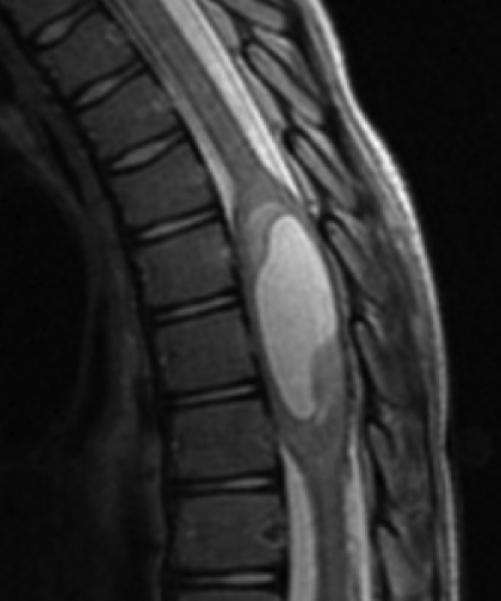
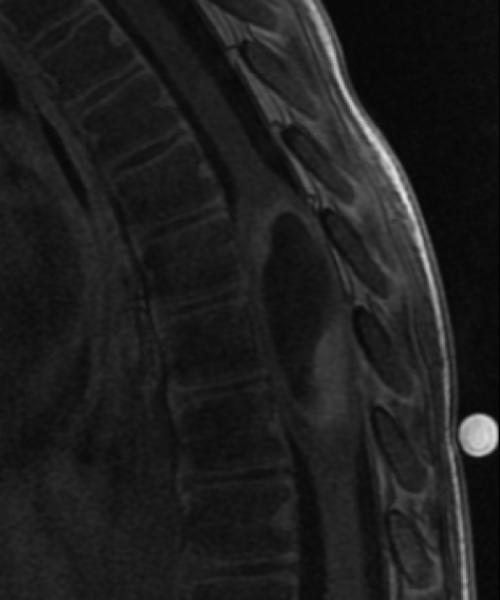
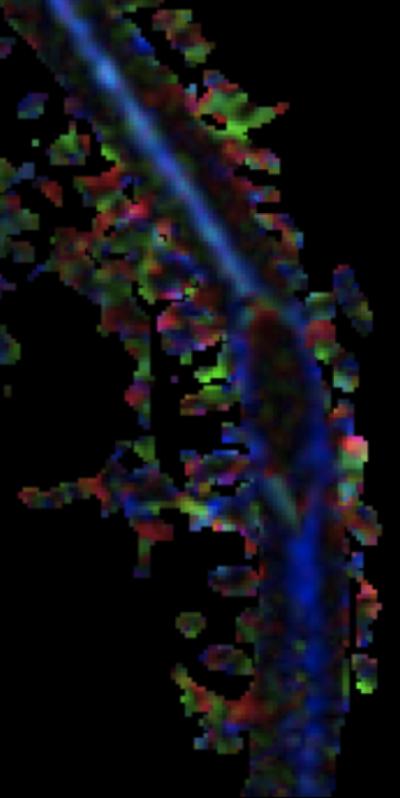
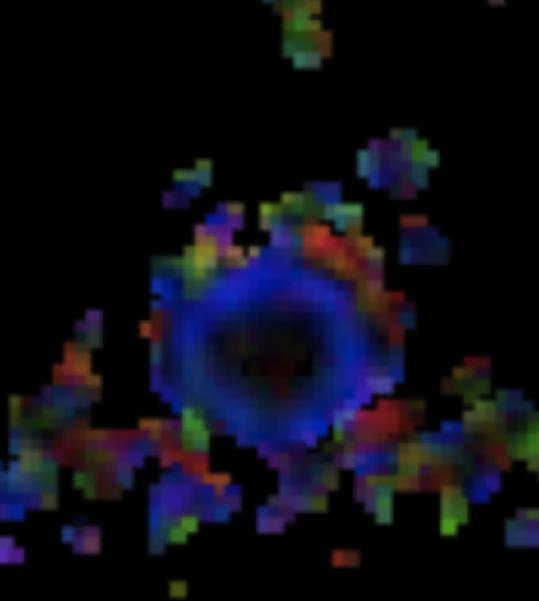
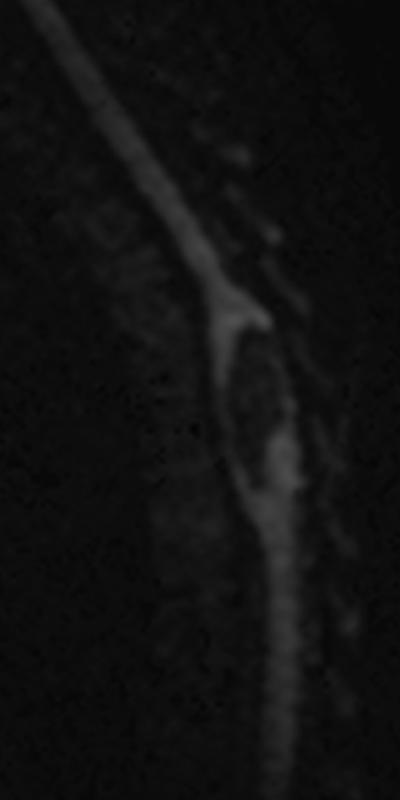
A: Sagittal T2W image of the mid-thoracic spinal cord shows a cystic ISCN spanning the T7 through T9 levels. B: Sagittal T1W post-contrast image shows a thin peripheral rim of enhancement with a nodule along the posterior/inferior aspect and focal thickening along the anterior/superior aspect of the cyst. C: Sagittal trace image from diffusion tensor imaging. Sagittal (D) and axial (E) DE-FA map of the thoracic cord. F: Lateral projection DT-FT image showing continuity of fiber tracts from above to below the mass, which are peripherally deviated as they traverse the lesion
Figure 2.
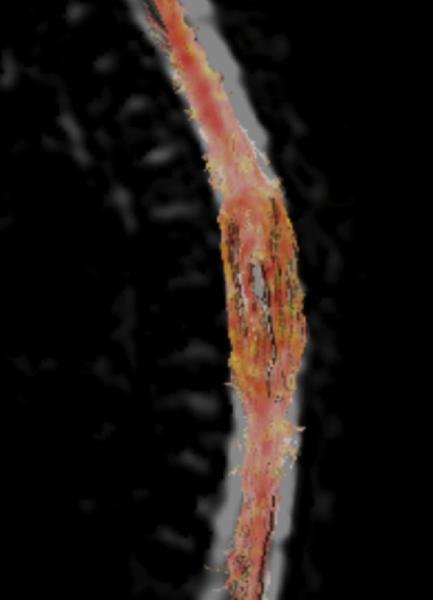
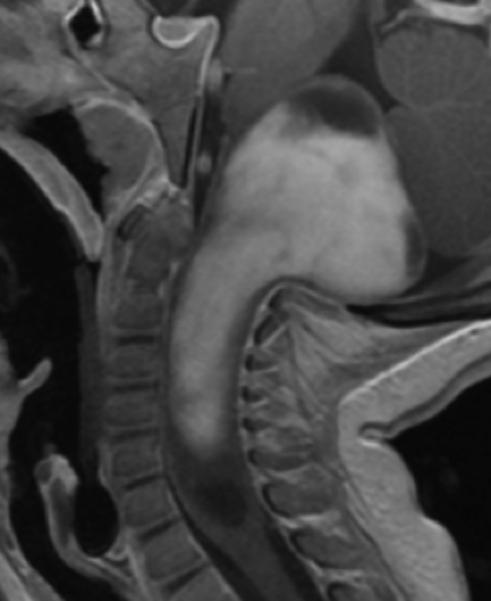
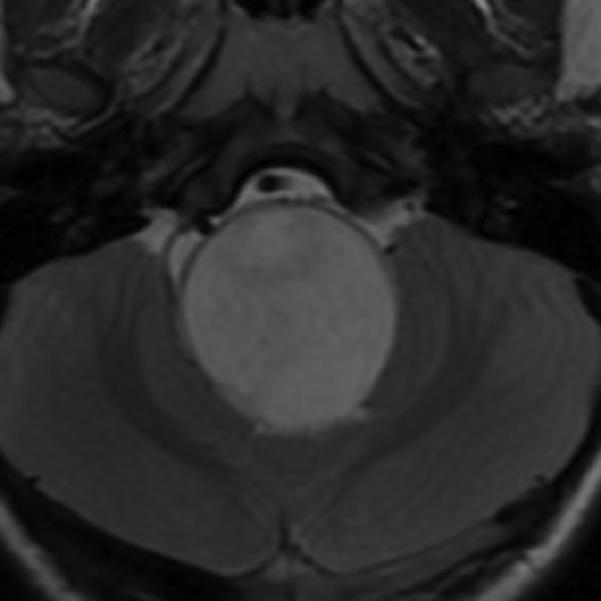
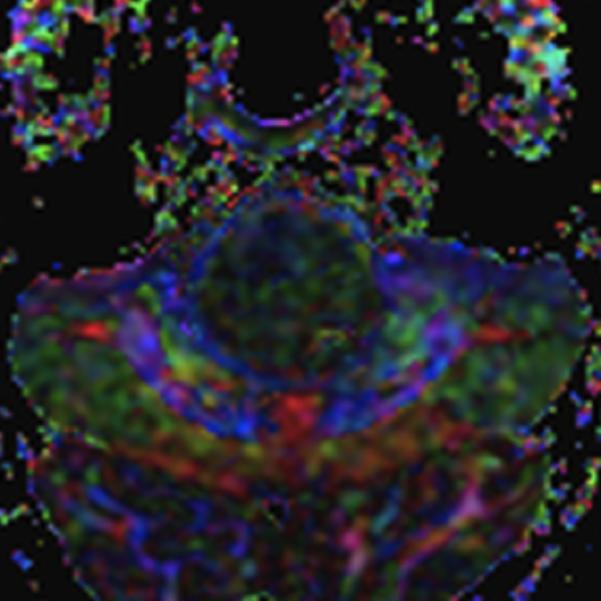
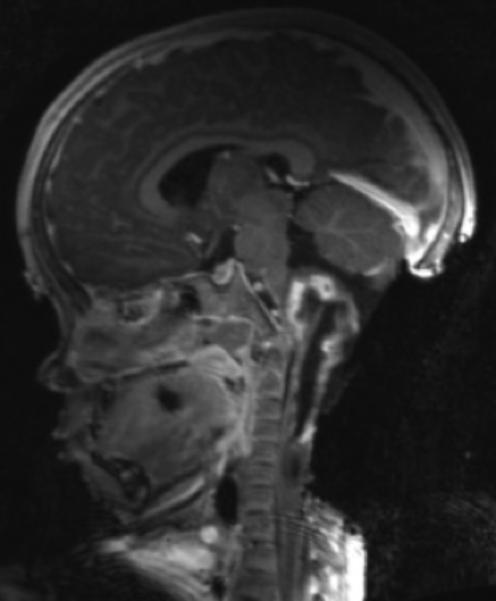
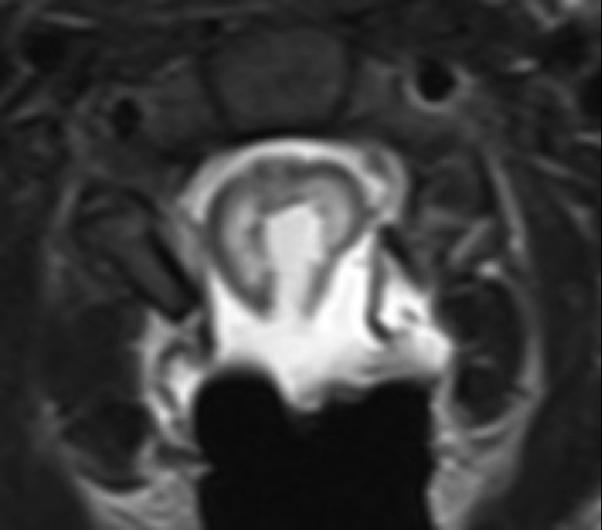
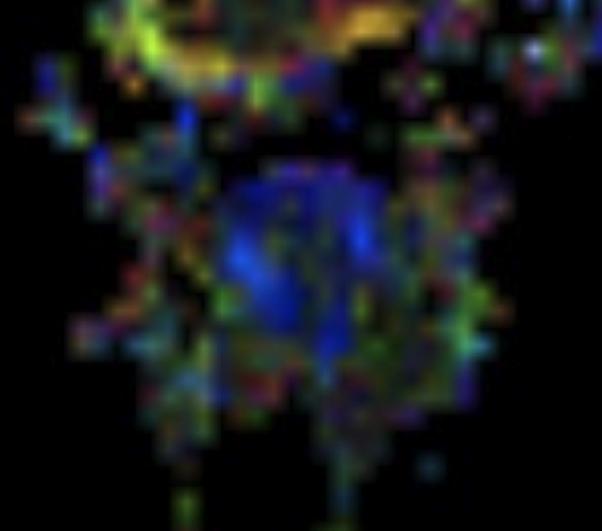
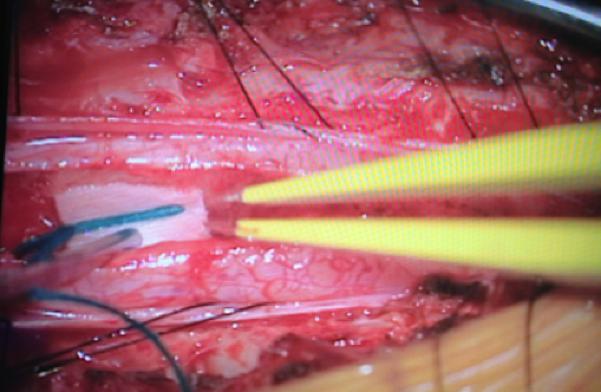
A: Sagittal T1 post-gadolinium image of the craniocervical junction shows an expansile intramedullary mass involving the mid- and upper cervical cord, with cephalad extension into the medulla oblongata. B: Axial T2WI at the level of the inferior medulla oblongata shows a thin hypointense rim along the anterior margin of the lesion, containing white matter fibers directed in a craniocaudal direction on DE-FA (C). D: Sagittal T1 post-gadolinium image from intraoperative MRI shows subtotal resection of the mass with decreased mass effect; however, there are residual nodular areas of enhancement at the superior margins. E: Axial T2W image from intraoperative MRI shows a dorsal myelotomy with T2 prolongation within the central cord soft tissue and a peripheral thin rim of hypointense tissue. The surgeons decided to discontinue further resection of tumor, as it became difficult to differentiate edematous re-expanding tissue from neoplasm, with the goal of treatment with either adjunctive therapy or observation with possible continued resection in approximately 1-2 years. F: Axial DE-FA from intraoperative MRI shows a craniocaudal diffusion orientation within the peripheral hypointense rim and portions of the areas with T2 prolongation, confirming suspicion that there were intact white matter tracts within the central cord signal abnormality, giving further support to the decision to discontinue further resection. No post-operative neurologic deficit was noted. G: Intraoperative image showing resection through a dorsal myelotomy, at which time a discrete margin was identified.
Figure 3.

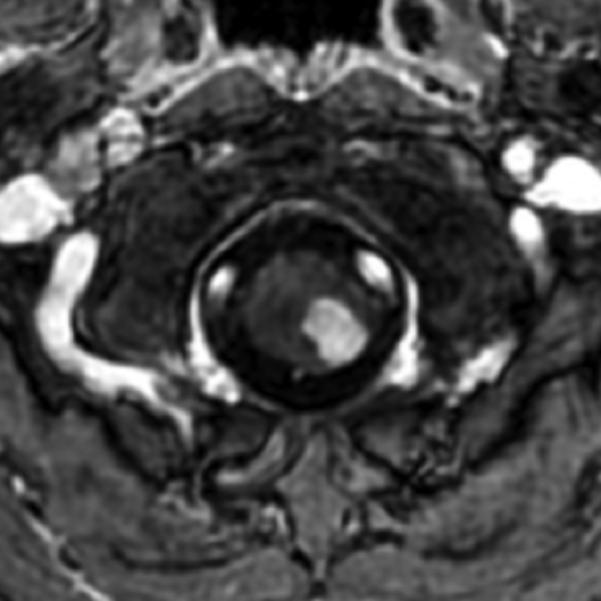
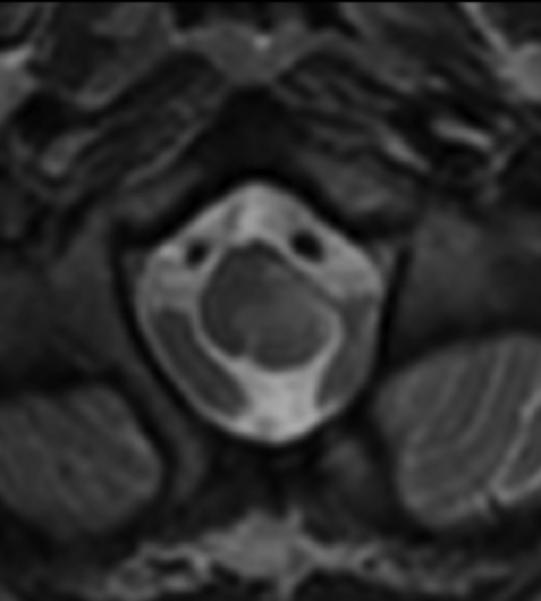
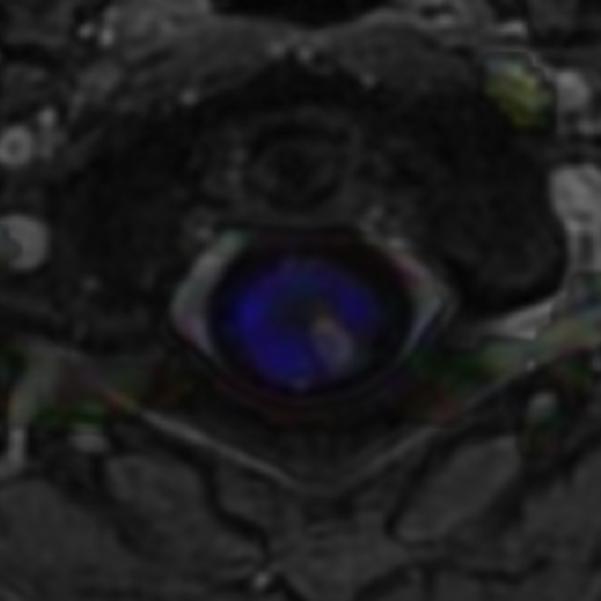

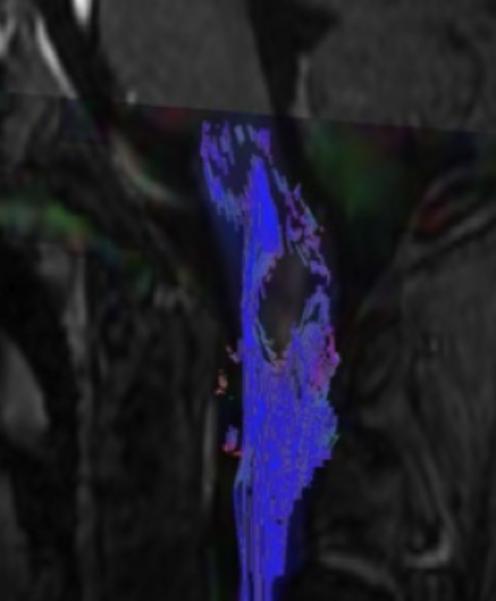
Sagittal (A) and axial (B) T1W image of the upper cervical cord shows an enhancing intramedullary mass in the left dorsolateral aspect of the spinal cord immediately caudal to the foramen magnum. Axial T2W image (C) at the superior aspect of the lesion shows expansion of the left dorsolateral aspect of the upper cervical cord with T2 prolongation extending beyond the margins of the enhancement. Axial T1W post-gadolinium image at the same level with DE-FA overlay (D) shows diminished FA in the left dorsolateral aspect of the cord, corresponding to the expected location of the spinal trigeminal tract and causing the ipsilateral jaw pain. Coronal (E) and sagittal (F) T1W post-contrast images with DT-FT overlay show disruption of fibers by the mass.
In all 8 patients with DTI evidence of splaying of fibers a discrete dissection plane was found at the margin of the tumor in at least some locations, however along margins where the cord parenchyma was thinner than 1 mm there were edematous changes in the cord parenchyma which necessitated caution during resection. In both patients with DTI evidence of tumor infiltration who underwent open biopsy, no discrete tumor margin could be identified under the operating microscope.
DISCUSSION
Diffusion tensor imaging and DT-FT can be performed in pediatric intramedullary spinal cord neoplasms as an adjunct to conventional structural imaging to help determine the location of tumor margins as well as the presence and degree of deflection of white matter tracts. Unlike preliminary results in adult intramedullary spinal cord neoplasms, DTI of ISCN in children does not differentiate astrocytomas from ependymomas. The pilocytic astrocytomas seen in seven of the 10 children in this series have histologic and structural differences from astrocytomas seen in prior adult spinal cord DTI series. Diffusion tensor imaging does appear to document whether there is splaying versus disruption of fibers, allowing determination of whether it is safe to attempt resection or whether biopsy is more appropriate. These findings were concordant with intraoperative findings by the neurosurgeon. Therefore, the true role of spinal cord DTI and DT-FT may not be in predicting histology, but rather in determining resectability.
Diffusion tensor imaging and DT-FT have proven to be very useful in evaluating white matter tracts, with nearly the entirety of this experience being within the brain. Because of the absence of a true “gold standard,” DTI and DT-FT have been difficult to fully validate. However, the growing body of literature demonstrating its accuracy and utility in tumors, vascular lesions, and surgery for epilepsy adds to the confidence in using this technique.
More recently, this technology has been applied to the spinal cord [8, 11, 12], including presurgical planning of adult spinal cord tumor procedures [7]. Preliminary work has suggested that DTI of adult ISCN can differentiate astrocytomas from ependymomas [7-9]. In adults, infiltrative astrocytomas are more common than in the pediatric population, while ependymomas tend to be well circumscribed. These tumors could be characterized using DTI and DT-FT to evaluate the structural integrity of the white matter tracts, which could be a way to decrease the need for biopsy of the adult spinal cord tumor, or at least to allow the primary surgical procedure to be attempts at resection in patients with suspected ependymomas.
Histologic information from ISCN is important in determining the type and goal of treatment. In adults, astrocytomas are at high risk of poorer outcomes from aggressive surgery (5). Spinal cord ependymomas in adults and intramedullary spinal cord tumors in children are primarily surgically resected (4). For infiltrative and high-grade ISCN adjuvant therapy is typically utilized, as resection is not possible (6). The literature has shown that gross-total resection and subtotal resection yield better patient outcomes than partial tumor resection; however, one of strongest predictors of patient outcome is preoperative neurological status [13, 14].
Additional studies are needed to evaluate the clinical usefulness of DTI and DT-FT in pediatric spinal cord tumors. The histopathological spectrum of intramedullary pediatric spinal cord tumors is different from adult intramedullary spinal cord tumors. Pediatric spinal cord astrocytomas are often pilocytic, which is rare in the adult central nervous system. Pilocytic astrocytomas tend to be circumscribed and discrete, while the astrocytomas seen in adults tend to be infiltrative lesions such as fibrillary astrocytoma or high-grade astrocytomas, including glioblastoma and anaplastic astrocytoma. These differences result in different imaging characteristics. We hypothesize that because of the discrete nature of most pediatric ISCN, DTI is less likely to differentiate astrocytoma from ependymoma in children compared to adults. However, this imaging would allow surgeons to determinate the level of infiltration of the tumor and help pre-surgical planning and counseling. Determining the extent of tumor infiltration by using DTI and DT-FT will allow for increased opportunities for safe total and subtotal tumor resection with less possibility for morbidity.
One of the major technical difficulties in spinal DTI is susceptibility artifact from structures including, but not limited to, bone and air, which are in closer proximity to the spinal cord than to the brain, especially in the thoracic spine. Another challenge in spinal DTI acquisition and interpretation is because of the smaller size of the spinal cord compared to the larger size of the brain. In the brain, different pathways run in different directions and connect in various orientations. In the spinal cord, a majority of fiber tracts are parallel in the craniocaudal direction. Diffuse tensor imaging has lower spatial resolution than most other imaging techniques. Other limitations of this report are due to the relatively small size of the case series and heterogeneity of tumors. Some tumors may also hemorrhage, which contributes to artifactual imaging. Some are tumors in the thoracic region and adjacent to lungs. The air in the lungs causes artifact. Another limitation is motion caused by respiration and by children moving during imaging.
Future work will include standardizing acquisition and analysis techniques for DTI of the spinal cord, as processing algorithms have typically been optimized for intracranial DTI. Standardization, however, has proven difficult even for intracranial DTI, for which there is more research and clinical experience than in the spinal cord. If pediatric neuro-oncology centers can standardize, at least to some degree, DTI acquisition in suspected ISCN, it may allow for a multi-institution registry to further characterize this rare entity.
Our experience with DTI and DT-FT suggests that these modalities are feasible ways to assess white matter tracts in pediatric intramedullary spinal cord tumor patients. The assessment of white matter tracts aids the surgeon in preoperative planning and patient counseling. Based on our small series, spinal cord DTI and DT-FT could contribute to better patient outcomes in the pediatric population.
CONCLUSIONS
Diffusion tensor imaging of pediatric ISCN can assist in surgical planning by determining whether resection, de-bulking or biopsy is appropriate, while minimizing iatrogenic neurologic injury.
Footnotes
Conflict of interest statement:
We declare that we have no conflict of interest.
Contributor Information
Asim F. Choudhri, Le Bonheur Children's Hospital, Department of Radiology, University of Tennessee Health Science Center, 848 Adams Ave-G216, Memphis, TN 38103, USA; Le Bonheur Neuroscience Institute, Le Bonheur Children's Hospital, Memphis, TN, USA.
Matthew T. Whitehead, Le Bonheur Children's Hospital, Department of Radiology, University of Tennessee Health Science Center, 848 Adams Ave-G216, Memphis, TN 38103, USA Le Bonheur Neuroscience Institute, Le Bonheur Children's Hospital, Memphis, TN, USA.
Paul Klimo, Jr, Le Bonheur Neuroscience Institute, Le Bonheur Children's Hospital, Memphis, TN, USA; Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, TN, USA.
Blake K. Montgomery, School of Medicine, University of Missouri-Kansas City, Kansas City, MO, USA
Frederick A. Boop, Le Bonheur Neuroscience Institute, Le Bonheur Children's Hospital, Memphis, TN, USA Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, TN, USA.
References
- 1.Asirvatham JR, Deepti AN, Chyne R, et al. Pediatric tumors of the central nervous system: a retrospective study of 1,043 cases from a tertiary care center in South India. Childs Nerv Syst. 2011;27:1257–1263. doi: 10.1007/s00381-011-1407-z. doi: 10.1007/s00381-011-1407-z. [DOI] [PubMed] [Google Scholar]
- 2.Pillai JJ, Zaca D, Choudhri A. Clinical impact of integrated physiologic brain tumor imaging. Technol Cancer Res Treat. 2010;9:359–380. doi: 10.1177/153303461000900406. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Kim JH, Park JB, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol. 2011;40:1543–1551. doi: 10.1007/s00256-011-1161-z. doi: 10.1007/s00256-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 4.Phillips NS, Sanford RA, Helton KJ, et al. Diffusion tensor imaging of intraaxial tumors at the cervicomedullary and pontomedullary junctions. Report of two cases. J Neurosurg. 2005;103:557–562. doi: 10.3171/ped.2005.103.6.0557. doi: 10.3171/ped.2005.103.6.0557. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed FB, Hunter LN, Barakat N, et al. Diffusion tensor imaging of the pediatric spinal cord at 1.5T: preliminary results. AJNR Am J Neuroradiol. 2011;32:339–345. doi: 10.3174/ajnr.A2334. doi: 10.3174/ajnr.A2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulcahey MJ, Samdani A, Gaughan J, et al. Diffusion tensor imaging in pediatric spinal cord injury: preliminary examination of reliability and clinical correlation. Spine. 2012;37:E797–803. doi: 10.1097/BRS.0b013e3182470a08. doi: 10.1097/BRS.0b013e3182470a08. [DOI] [PubMed] [Google Scholar]
- 7.Setzer M, Murtagh RD, Murtagh FR, et al. Diffusion tensor imaging tractography in patients with intramedullary tumors: comparison with intraoperative findings and value for prediction of tumor resectability. Journal of neurosurgery Spine. 2010;13:371–380. doi: 10.3171/2010.3.SPINE09399. doi: 10.3171/2010.3.SPINE09399. [DOI] [PubMed] [Google Scholar]
- 8.Thurnher MM, Law M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am. 2009;17:225–244. doi: 10.1016/j.mric.2009.02.004. doi: 10.1016/j.mric.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Ducreux D, Lepeintre J-F, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006;27:214–216. [PMC free article] [PubMed] [Google Scholar]
- 10.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Singhi S, Tekes A, Thurnher M, et al. Diffusion tensor imaging of the maturing paediatric cervical spinal cord: From the neonate to the young adult. J Neuroradiol. 2012;39:142–148. doi: 10.1016/j.neurad.2011.05.002. doi: 10.1016/j.neurad.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Semin Roentgenol. 2006;41:294–311. doi: 10.1053/j.ro.2006.07.003. doi: 10.1053/j.ro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Jallo GI, Freed D, Epstein F. Intramedullary spinal cord tumors in children. Childs Nerv Syst. 2003;19:641–649. doi: 10.1007/s00381-003-0820-3. doi: 10.1007/s00381-003-0820-3. [DOI] [PubMed] [Google Scholar]
- 14.Jallo GI, Kim BS. The current management of intramedullary neoplasms in children and young adults. Ann Neurosurg. 2001 [Google Scholar]