Abstract
Objectives. We examined the association between trajectories of partnership status over the life course and objectively measured health indicators in midlife.
Methods. We used data from 4 waves (1981, 1991, 2000, and 2002–2004) of the British National Child Development Study (NCDS), a prospective cohort study that includes all people born in Britain during 1 week in March 1958 (n = 18 558).
Results. After controlling for selection attributable to early-life and early-adulthood characteristics, we found that life-course trajectories of partnership status were associated with hemostatic and inflammatory markers, the prevalence of metabolic syndrome and respiratory function in midlife. Never marrying or cohabiting was negatively associated with health in midlife for both genders, but the effect was more pronounced in men. Women who had married in their late 20s or early 30s and remained married had the best health in midlife. Men and women in cohabiting unions had midlife health outcomes similar to those in formal marriages.
Conclusions. Partnership status over the life course has a cumulative effect on a wide range of objectively measured health indicators in midlife.
Numerous studies have found that married people have better health and lower mortality than unmarried people, and these findings have been replicated in different countries and time periods.1–18 A reduction in health inequalities related to marital status therefore has the potential to shift the distribution of risk and improve population health.19 However, to do so, further understanding of the mechanisms that link marital status and health is needed, including further consideration of health-related selection into various marital statuses, the operation of health-protective effects of marriage, and the accumulation of benefits and risks of marital status trajectories over the life course.
With a few exceptions,20 studies of marital status and health have considered only current marital status or transitions over relatively short periods,15 and only a few studies have considered the association between nonmarital cohabitation and health,12,21 a topic of increasing importance given that cohabitation is becoming more common in the United Kingdom.22 Furthermore, of those studies that have used measures of health, rather than mortality, as an outcome,23 most have used self-reported measures. In the few studies in which objective health indicators were used, sample sizes were relatively small.24,25
In this study, we used data from a population-based birth cohort to summarize longitudinal patterns of partnership status that distinguish marital status and nonmarital cohabitation. We used a model-based approach that allowed us to capture stability as well as transitions in partnership status over a 21-year period (ages 23–44 years) and used this to investigate the effects that 21-year trajectories of partnership status have on a wide range of biomarkers in midlife. Our objective was to investigate the association of different trajectories of partnership status over the life course and objectively measured health indicators in midlife.
METHODS
We used data from the British National Child Development Study, a birth cohort study that includes all people born in Britain during 1 week in March 1958. Cohort members have been followed up periodically from birth into adulthood.26 To derive the partnership status trajectories, we used data from 4 sweeps of the study: 1981 (n = 12 537), 1991 (n = 11 469), 2000 (n = 11 419), and 2002–2004 (n = 9377), when study members were aged 23, 33, 42, and 44 to 46 years, respectively. Our outcomes were derived from the 2002 to 2004 clinical examination that was carried out at participants’ homes by 122 specially trained nurses. To control for possible selection effects, we used information from earlier sweeps carried out between 1958 and 1974 (when study members were aged 0 to 16 years). Our analytic sample included participants with at least 3 valid responses on the marital status and cohabitation indicators and complete information on the background confounders described later (n = 10 226; 5256 women, 4970 men).
Measures
Indicators of partnership status.
We used binary indicators representing whether a participant was married or was cohabiting with someone to whom they were not married at each measurement wave. Each of the 4 measurement waves is thus represented by 2 indicators (1 for marital status and 1 for cohabitation). We also included in the model information on whether participants had remarried by age 44 years (Table 1).
TABLE 1—
Frequency Distribution of Marital Status and Nonmarital Cohabitation Indicators: British National Child Development Study, 1981, 1991, 2000, and 2002–2004
| Married |
Cohabiting |
|||
| Men, % | Women, % | Men, % | Women, % | |
| Aged 23 y | ||||
| Noa | 65.2 | 45.6 | 94.6 | 92.8 |
| Yes | 34.8 | 54.4 | 5.4 | 7.2 |
| Aged 33 y | ||||
| No | 31.0 | 27.9 | 89.2 | 90.4 |
| Yes | 69.0 | 72.1 | 10.8 | 9.6 |
| Aged 40 y | ||||
| No | 29.4 | 28.9 | 90.5 | 91.1 |
| Yes | 70.6 | 71.1 | 9.5 | 8.9 |
| Aged 42 y | ||||
| No | 27.0 | 28.9 | 87.8 | 87.9 |
| Yes | 73.0 | 71.1 | 12.2 | 12.1 |
| Remarried | ||||
| No | 90.8 | 88.7 | ||
| Yes | 9.2 | 11.3 | ||
For cohabiting, “no” responses include all other partnership status categories (married, single, divorced, widowed).
Biomarkers in midlife.
We used 5 hemostatic and inflammatory markers measured at ages 44 to 46 years (2002–2004 sweep): C-reactive protein, fibrinogen,27 fibrin d-dimer,28 von Willebrand factor, and tissue plasminogen activator antigen.29 Metabolic syndrome was characterized by the standard International Diabetes Federation definition (see Appendix I, available as a supplement to the online version of this article at http://www.ajph.org). We also used forced vital capacity (FVC), a marker of respiratory functioning. Further details of the laboratory procedures are available elsewhere.30,31
Confounders.
To control for possible selection processes into partnership status, we included various early-life and early-adulthood (age 23 years) characteristics in our models. In the existing literature, selection into partnership status has been found to be influenced by income, educational attainment, and past and current health status.10,32 In our models, we adjusted for early-life socioeconomic position using a latent summary of serious financial hardship during the past year at age 11 years, access to household amenities at age 11 years, paternal social class at age 7 years, number of people per room at age 7 years, housing tenure at age 7 years, and paternal weekly net pay at age 16 years (further details on the derivation of the latent summary are presented in Appendix V, available as a supplement to the online version of this article at http://www.ajph.org). Health center attendance during the previous year at age 16 years, disability at age 16 years, and height at age 7 years were used as indicators of early-life health status. General cognitive ability measured at age 11 years was used as an indicator of early-life cognitive ability.
We also controlled for variables measured at age 23 years: educational attainment, social class, housing tenure, net family income, self-rated health, depression, employment status, body mass index and presence of long-standing disability. Current use of medication and lab processing–related variables were also included in the analyses. We did not control for variables such as income, number of children, and employment status that were available for sweeps between ages 23 and 42 years because they may lay on the causal pathway that links the partnership status typology with midlife biomarkers.
Statistical Modeling
We used latent class analysis to derive a longitudinal typology of partnership status. In latent class analysis, longitudinal trajectories are unknown but can be inferred from patterns of responses on observed indicators of marital status and cohabitation measured over time. We found 325 unique response patterns for men and 316 for women. In this instance, we used latent class analysis to summarize these patterns by creating longitudinal profiles—trajectories—in a parsimonious way. This approach can be viewed as an evidence-based approximation that improves a researcher’s ability to identify, summarize, and communicate complex patterns in longitudinal data33 and has been used in a wide range of applications.34–36 We used the derived longitudinal typology to investigate the association between trajectories of partnership status with a wide range of biomarkers in midlife. C-reactive protein, fibrinogen, fibrin d-dimer, tissue plasminogen activator antigen, and von Willebrand factor were log transformed to normalize their distributions and FVC was left untransformed before we performed linear regression analyses. Metabolic syndrome was modeled as a binary outcome with logistic regression.
Selection bias, in the form of incomplete or missing data, is almost ubiquitous in the observational setting of the National Child Development Study, and it is well known that unbiased estimates cannot be obtained without properly addressing the implications of incompleteness. We used the full information maximum likelihood method, which is naturally incorporated into the generalized latent variable modeling framework.37 In this full likelihood context model, parameters and standard errors are estimated directly from the available data, under the assumption that missingness is at random and that the models are correctly specified.38,39 In our analyses, assuming missingness is at random means that all the variables that may plausibly be responsible for the missing data-generating mechanism are complete and are included in the model as explanatory variables or intermediate outcomes. These variables were early life socioeconomic position latent summary, health center attendance during the past year at age 16 years, disability at age 16 years, height at age 7 years, cognitive ability at age 11 years, educational attainment at age 23 years, smoking status at age 23 years, self-rated health at age 23 years, depression at age 23 years, employment status at age 23 years, social class at age 23 years, housing tenure at age 23 years, net family income at age 23 years, body mass index at age 23 years, presence of long-standing disability at age 23 years, and current use of medication at age 42 years.
Any other missingness that is not accounted for by these variables was assumed to be completely at random because we assumed that all systematic causes of missingness had been accounted for. We believe that this assumption is reasonable because socioeconomic position and age have been shown to be the main drivers of selection attributable to attrition in population surveys in the United Kingdom.40,41 All models were estimated with the Mplus seventh edition software (Muthén and Muthén,42 Los Angeles, CA), with the robust maximum likelihood estimator and Monte Carlo integration.
RESULTS
In Table 2, we present information criteria, likelihood-based tests, and the entropy coefficient, a measure of classification quality (values close to 1 indicate good allocation quality and low classification error) for different specifications of the latent class model. As expected, model fit improved with each additional class for both men and women. The classification quality as indicated by the entropy was highly satisfactory for all models. Because all bootstrapped likelihood ratio tests returned significant P values, model selection was based on relative fit and substantive criteria. As can be observed in Table 2, as well as Figures A and B in Appendix II (available as a supplement to the online version of this article at http://www.ajph.org), the difference between models in all information criteria becomes smaller from the 6-class model onward for both men and women, indicating that 6 to 8 classes adequately describe the data. Closer inspection of the derived classes revealed that the additional seventh and eighth classes largely replicated the patterns of already existing classes, but with a very small prevalence (< 1% for men and < 2% for women). We therefore selected 6-class models for both men and women as the most parsimonious description of the longitudinal patterns in the data.
TABLE 2—
Log-Likelihood and Information Criteria for Alternative Latent Class Models: British National Child Development Study, 1981, 1991, 2000, and 2002–2004
| No. of Classes | Parameters | Log-Likelihood | AIC | BIC | ssa BIC | Entropy | BLRT | P |
| Men | ||||||||
| 1 | 9 | −18 503.599 | 37 025.197 | 37 083.798 | 37 055.199 | 1.000 | ||
| 2 | 19 | −15 402.771 | 30 843.543 | 30 967.255 | 30 906.880 | 0.927 | 6 201.655 | .001 |
| 3 | 29 | −14 821.097 | 29 700.194 | 29 889.018 | 29 796.867 | 0.947 | 1 163.348 | .001 |
| 4 | 39 | −14 548.412 | 29 174.824 | 29 428.760 | 29 304.831 | 0.932 | 545.371 | .001 |
| 5 | 49 | −14 296.983 | 28 691.966 | 29 011.014 | 28 855.309 | 0.909 | 502.857 | .001 |
| 6 | 59 | −14 176.065 | 28 470.130 | 28 854.289 | 28 666.807 | 0.922 | 241.837 | .001 |
| 7 | 69 | −14 072.910 | 28 283.821 | 28 733.092 | 28 513.834 | 0.925 | 206.309 | .001 |
| 8 | 79 | −13 990.324 | 28 138.648 | 28 653.031 | 28 401.997 | 0.912 | 165.172 | .001 |
| 9 | 89 | −13 943.867 | 28 065.734 | 28 645.229 | 28 362.418 | 0.920 | 92.914 | .001 |
| 10 | 99 | −13 909.547 | 28 017.095 | 28 661.701 | 28 347.114 | 0.923 | 68.639 | .001 |
| Women | ||||||||
| 1 | 9 | −20 063.467 | 40 144.933 | 40 204.037 | 40 175.438 | 1.000 | ||
| 2 | 19 | −16 423.428 | 32 884.855 | 33 009.631 | 32 949.255 | 0.944 | 7 280.078 | .001 |
| 3 | 29 | −15 816.767 | 31 691.533 | 31 881.980 | 31 789.827 | 0.962 | 1 213.322 | .001 |
| 4 | 39 | −15 529.946 | 31 137.891 | 31 394.009 | 31 270.080 | 0.941 | 573.642 | .001 |
| 5 | 49 | −15 306.326 | 30 710.653 | 31 032.442 | 30 876.736 | 0.918 | 447.239 | .001 |
| 6 | 59 | −15 103.758 | 30 325.516 | 30 712.976 | 30 525.494 | 0.905 | 405.137 | .001 |
| 7 | 69 | −15 003.290 | 30 144.579 | 30 597.711 | 30 378.451 | 0.917 | 200.937 | .001 |
| 8 | 79 | −14 917.217 | 29 992.434 | 30 511.237 | 30 260.201 | 0.920 | 172.145 | .001 |
| 9 | 89 | −14 856.621 | 29 891.242 | 30 475.716 | 30 192.903 | 0.928 | 121.192 | .001 |
| 10 | 99 | −14 813.435 | 29 824.869 | 30 475.015 | 30 160.425 | 0.916 | 86.334 | .001 |
Note. AIC = Akaike information criterion; BIC = Bayesian information criterion; BLRT = bootstrapped likelihood ratio test comparison for N vs N − 1 class models; ssa BIC = sample-size-adjusted Bayesian information criterion.
Although the number of classes was the same for both genders, the prevalence and composition of the latent longitudinal typologies differed. The probabilities of being married, cohabiting, and remarrying, conditional on group membership, are presented in Figure 1 (men) and Figure 2 (women), as well as in Tables A and B in Appendix III (available as a supplement to the online version of this article at http://www.ajph.org). In men, the first and most prevalent class (n = 3073; 61.8%) consisted of men who married in their 20s or early 30s and remained married, with this generally being their only marriage. The second class (n = 411; 8.3%) was characterized by men who married in their 20s or early 30s but later divorced, with increasing cohabitation but little remarriage by their 40s. The third class (n = 373; 7.5%) included men who mostly never married but who cohabited from their late 20s or early 30s onward. The fourth class (n = 467; 9.4%) included men who married in their mid- or late 30s, preceded by cohabitation in their early 30s for many, and who then remained married. The fifth class (n = 94; 1.9%) was characterized by men who divorced in their mid- or late 30s but who later remarried, with many cohabiting in between. Finally, the sixth class (n = 553; 11.1%) consisted almost exclusively of men who never married and never cohabited.
FIGURE 1—
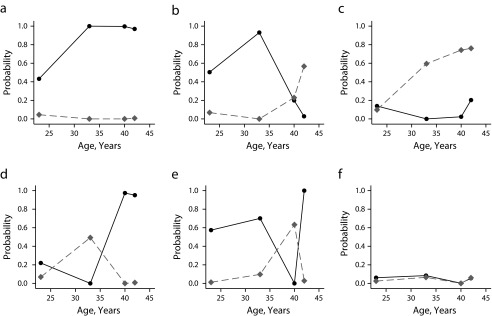
Longitudinal typologies of probability of marriage (solid black line) and cohabitation (dotted gray line) among men in (a) class 1, (b) class 2, (c) class 3, (d) class 4, (e) class 5, and (f) class 6: British National Child Development Study, 1981, 1991, 2000, and 2002–2004.
Note: Class 1: n = 3073 (61.8%), remarried probability = 0.123. Class 2: n = 411 (8.3%), remarried probability = 0.181. Class 3: n = 373 (7.5%), remarried probability = 0.039. Class 4: n = 467 (9.4%), remarried probability = 0.379. Class 5: n = 94 (1.9%), remarried probability = 0.917. Class 6: n = 553 (11.1%), remarried probability = 0.023.
FIGURE 2—
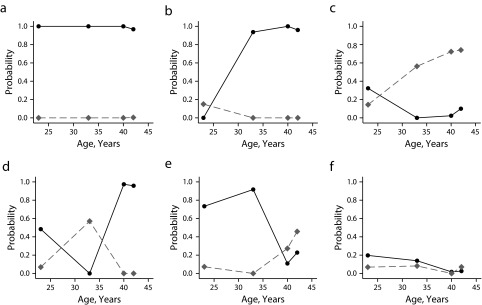
Longitudinal typologies of probability of marriage (solid black line) and cohabitation (dotted gray line) among women in (a) class 1, (b) class 2, (c) class 3, (d) class 4, (e) class 5, and (f) class 6: British National Child Development Study, 1981, 1991, 2000, and 2002–2004.
Note. Class 1: n = 2209 (42.0%), remarried probability = 0.138. Class 2: n = 1215 (23.1%), remarried probability = 0.123. Class 3: n = 429 (8.1%), remarried probability = 0.085. Class 4: n = 294 (5.6%), remarried probability = 0.661. Class 5: n = 457 (8.7%), remarried probability = 0.312. Class 6: n = 652 (12.4%), remarried probability = 0.028.
In women, the most prevalent class (n = 2209; 42%) consisted of women who married by their early 20s, with this usually being their only marriage up to age 44 years. The second class (n = 1215; 23.1%) was characterized by women who married in their late 20s or early 30s, with this being their only marriage until age 44 years. In the third class (n = 429, 8.1%) were women who never married or married in their 20s and subsequently separated without remarrying, and who were more likely to cohabit from their early 30s onward. The fourth class (n = 294; 5.6%) was characterized by women who got married and subsequently divorced in their 20s or early 30s, cohabited, and then remarried. Women allocated to the fifth class (n = 457; 8.7%) married by their 20s or early 30s but divorced in their mid- to late 30s, with some later cohabiting or remarrying. The sixth class (n = 652; 12.4%) consisted almost entirely of women who never married or cohabited.
In Table 3, we present the parameter estimates (linear regression coefficients and odds ratios [ORs], where appropriate) and corresponding 95% confidence intervals (CIs) that capture the association between the longitudinal partnership status typology and biomarkers in midlife. Men assigned to class 6 had worse health outcomes than the reference group (men who were married in their 20s or early 30s and who had remained married ever since, i.e., Class 1). They had higher fibrinogen (b = 0.034; 95% CI = 0.011, 0.056), C-reactive protein (b = 0.147; 95% CI = 0.024, 0.270), and tissue plasminogen activator antigen (b = 0.061; 95% CI = 0.006, 0.116) and lower FVC (b = −0.132; 95% CI = −0.227, −0.037). Men assigned to class 2 (divorced by their late 30s but who did not remarry) were less likely to have metabolic syndrome than the reference group (OR = 0.754; 95% CI = 0.575, 0.988). Men who were not married but who had cohabited since their late 20s or early 30s (class 3), had lower FVC than the reference group (b = −0.103; 95% CI = −0.206, −0.001). There was evidence of effect modification by early-life health and early-life socioeconomic position indicators with respect to fibrinogen, C-reactive protein, and FVC. The observed associations of the longitudinal typology were more pronounced in men who were healthy and financially comfortable during their childhood (results available from corresponding author).
TABLE 3—
Estimated Regression Parameters and 95% Confidence Intervals: British National Child Development Study, 1981, 1991, 2000, and 2002–2004
| Class | Fibrinogen, b (95% CI) | CRP, b (95% CI) | VWF, b (95% CI) | TPA, b (95% CI) | Ddimer, b (95% CI) | Metabolic Syndrome, OR (95% CI) | FVC, b (95% CI) |
| Men | |||||||
| 1 (Ref) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2 | 0.020 (−0.006, 0.045) | 0.128 (−0.009, 0.265) | 0.029 (−0.010, 0.069) | 0.036 (−0.029, 0.100) | 0.035 (−0.035, 0.106) | 0.754 (0.575, 0.988) | 0.077 (−0.020, 0.175) |
| 3 | 0.008 (−0.016, 0.032) | −0.022 (−0.169, 0.125) | −0.004 (−0.048, 0.041) | 0.022 (−0.045, 0.089) | 0.041 (−0.031, 0.112) | 1.075 (0.815, 1.417) | −0.103 (−0.206, −0.001) |
| 4 | 0.008 (−0.015, 0.031) | 0.007 (−0.113, 0.128) | 0.003 (−0.036, 0.041) | 0.003 (−0.054, 0.059) | 0.054 (−0.014, 0.123) | 1.077 (0.843, 1.376) | −0.080 (−0.172, 0.013) |
| 5 | 0.031 (−0.019, 0.082) | 0.080 (−0.210, 0.369) | 0.012 (−0.064, 0.088) | 0.061 (−0.066, 0.189) | 0.030 (−0.109, 0.169) | 0.666 (0.385, 1.152) | 0.020 (−0.165, 0.204) |
| 6 | 0.034 (0.011, 0.056) | 0.147 (0.024, 0.270) | 0.020 (−0.016, 0.057) | 0.061 (0.006, 0.116) | 0.039 (−0.029, 0.106) | 0.881 (0.690, 1.124) | −0.132 (−0.227, −0.037) |
| Women | |||||||
| 1 (Ref) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| 2 | −0.018 (−0.034, −0.001) | −0.085 (−0.184, 0.013) | −0.010 (−0.038, 0.017) | −0.035 (−0.080, 0.010) | −0.003 (−0.048, 0.043) | 1.011 (0.813, 1.256) | 0.056 (0.002, 0.110) |
| 3 | −0.011 (−0.036, 0.015) | −0.010 (−0.157, 0.136) | −0.002 (−0.045, 0.041) | 0.009 (−0.059, 0.078) | −0.065 (−0.130, 0.001) | 0.764 (0.551, 1.059) | 0.033 (−0.042, 0.108) |
| 4 | 0.010 (−0.018, 0.039) | 0.198 (0.033, 0.364) | 0.039 (−0.008, 0.085) | −0.026 (−0.110, 0.059) | −0.037 (−0.105, 0.031) | 1.075 (0.742, 1.560) | −0.002 (−0.101, 0.096) |
| 5 | −0.001 (−0.023, 0.023) | −0.033 (−0.175, 0.109) | −0.011 (−0.042, 0.029) | −0.008 (−0.072, 0.056) | 0.023 (−0.040, 0.085) | 0.694 (0.496, 0.972) | 0.022 (−0.051, 0.095) |
| 6 | 0.028 (0.006, 0.050) | 0.031 (−0.103, 0.165) | 0.023 (−0.013, 0.060) | −0.031 (−0.089, 0.028) | −0.014 (−0.073, 0.045) | 0.773 (0.580, 1.030) | −0.055 (−0.121, 0.010) |
Note. CI = confidence interval; CRP = C-reactive protein; Ddimer = fibrin d-dimer; FVC = forced vital capacity; OR = odds ratio; TPA = tissue plasminogen activator antigen; VWF = von Willebrand factor. Estimates were adjusted for serious early-life socioeconomic position (latent summary of financial hardship during the past year at age 11 years, access to household amenities at age 11 years, paternal social class at age 7 years, number of people per room at birth, housing tenure at age 7 years, and paternal weekly net pay at age 16 years), health center attendance during the past year at age 16 years, disability at age 16 years, height at age 7 years, cognitive ability at age 11 years, educational attainment at age 23 years, social class at age 23 years, housing tenure at age 23 years, net family income at age 23 years, self-rated health at age 23 years, depression at age 23 years, employment status at age 23 years, body mass index at age 23 years, presence of long-standing disability at age 23 years, current use of medication at age 42 years, and lab processing–related variables. All outcomes modeled with linear regression (with effect estimate reported as linear regression coefficient −b), except for metabolic syndrome for which logistic regression was used (with effect estimate reported as an OR).
A different pattern of associations emerged in women. Women assigned to class 6 (who never married or cohabited) had higher fibrinogen (b = 0.028; 95% CI = 0.006, 0.050) than did the reference group (class 1; those married in their early 20s and who were still married). Conversely, women assigned to class 2 (married in their late 20s or early 30s and who had remained married since) had the best health. Compared with the reference group (class 1), they had lower fibrinogen (b = −0.018; 95% CI = −0.034, −0.001) and higher FVC (b = 0.056; 95% CI = 0.002, 0.110). Women who married in their 20s or early 30s but divorced in their mid- to late 30s, many of whom later cohabited or remarried (class 5) were less likely to have metabolic syndrome than the reference group (OR = 0.694; 95% CI = 0.496, 0.972). We found evidence of effect modification by early-life health and early-life socioeconomic position indicators with respect to fibrinogen and FVC. The observed effects of the longitudinal typology were more pronounced in women who were healthy and financially comfortable during their childhood (results available from corresponding author).
DISCUSSION
A longitudinal typology of partnership status spanning 21 years was associated with a wide range of inflammatory and hemostatic markers as well as other objectively measured health outcomes in midlife. The observed effects differed between men and women, implying that the mechanisms that link partnership status and health may be gender specific. Among men, those who never married or cohabited had significantly higher levels on 3 hemostatic function biomarkers and worse respiratory function than men who were married and remained married for the duration of the observation period. This finding is largely in agreement with studies using self-reported health outcomes as well as studies on mortality.9,12,17,18,43 A different pattern of associations emerged in women. Those who married in their mid- to late 20s or early 30s and remained married for the whole observation period had the best health, with lower fibrinogen levels and better respiratory function than women who married in their early 20s. As expected from the previous literature, women who never married or cohabited had worse health than married women. However, this effect was only manifested in fibrinogen levels, indicating that not marrying or cohabiting is less detrimental among women than men or, as has been suggested, being married appears to be more beneficial to men.10,20,44–46
We found that with the exception of worse respiratory functioning in men, nonmarital cohabitation has similar effects to being married with respect to midlife health. This finding has implications for public health policy, considering the increasing number of individuals who choose to cohabit and not marry. Our results are in agreement with recent findings on self-rated health47 but contradict earlier findings on depression and self-reported physical health in the United States.48 Further research is required to shed more light on whether nonmarried cohabiters have worse health than those who are married. Differences between our findings and those of previous studies could be attributable to self-reporting bias in the latter or could reflect differences between the United Kingdom and the United States in the effect of nonmarital cohabitation on health (because in the United States being married is more strongly associated with socioeconomic position and race/ethnicity49–51) or differences in time periods considered. However, additional results from the National Child Development Study using self-rated health as the outcome support the former explanation—at least for cohabiting women—because they were more likely than married women to report that their health was poor or fair (OR = 1.408; 95% CI = 1.046, 1.898), although we observed no differences between the 2 groups in any of the biomarkers (results presented in Appendix VI, available as a supplement to the online version of this article at http://www.ajph.org).
It appears that for both genders transitions from and to marriage and nonmarital cohabitation do not have a detrimental effect on midlife health. We did not observe a difference in the biomarkers used in our study between participants who divorced and subsequently remarried or cohabited and those who were married for the duration of the observation period. We also found that men who divorced in their late 30s and did not subsequently remarry were less likely to suffer from metabolic syndrome in midlife. Both results are in accordance with previous findings, which have shown that after an initial decline in health, men tend to revert to predivorce health status.52
We found that trajectories of partnership status over 21 years are associated with a wide range of biomarkers in midlife. These effects were observed after controlling for factors that influence partnership status (selection) or both partnership status and health (confounding). In accordance with previous findings10,12,44,53,54 as well as with a recent UK study,32 we found evidence of selection mainly as a result of early-life socioeconomic position and early-life health but also as a result of educational attainment in early adulthood (results not presented here; available from the corresponding author). However, assuming that all sources of selection, confounding, and attrition were represented by variables included in our models, our finding that partnership status is associated with midlife health implies that this effect is independent of selection.
Several explanations of the mechanism that links partnership status and health have been proposed, including fertility history, social support, health-related behavior, and socioeconomic position.55–58 An added complexity in understanding the proposed mechanism is that these pathways may differ between longitudinal trajectories of partnership status and may also be gender specific. This analysis is beyond the scope of this article, but we will address these questions in a future study in which we will investigate the mechanism that underlies the association between the longitudinal partnership status typology and midlife biomarkers.
Strengths of this study are the inclusion of a wide range of biomarkers as health outcomes in midlife, the availability of data to control for well-known selection mechanisms, and the derivation of a longitudinal typology that allowed us to capture trajectories of partnership status over 21 years. However, several limitations should be considered when interpreting our results. We used observational data, and despite the wealth of the 1958 cohort, bias resulting from unknown or unmeasured confounders cannot be ruled out. Furthermore, our longitudinal typology captured the cumulative influence of different trajectories of partnership status on biomarkers in midlife. Thus, we were not able to investigate the short-term health effects of stressful events such as marital dissolution that have been suggested by the literature.11,59 Another important limitation is that our data on partnership status were based on self-report. Although the latent variable specification of our longitudinal typology deals with measurement error under certain assumptions for the measurement error mechanism, some bias resulting from social desirability may have influenced our results. Finally, we note that our results can be generalized only to those born in 1958 in the United Kingdom and perhaps to other cohorts born close to this year. Partnership status trajectories as well as the association between these and health outcomes may be different in other—especially younger—cohorts, and future research is needed to investigate these possibilities.
Acknowledgments
This work was supported by the Economic and Social Research Council, National Centre for Research Methods node “Pathways, Biosocial Influences to Health” (grants ES/I025561/1, ES/I025561/2, and ES/I025561/3).
Human Participant Protection
The National Child Development Study (1958 birth cohort) has ethical approval from various bodies in the United Kingdom for all the sweeps that were used in this study.
References
- 1.Cheung YB. Marital status and mortality in British women: a longitudinal study. Int J Epidemiol. 2000;29(1):93–99. doi: 10.1093/ije/29.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB, Sr, Benjamin EJ. Marital status, marital strain, and risk of coronary heart disease or total mortality: the Framingham Offspring Study. Psychosom Med. 2007;69(6):509–513. doi: 10.1097/PSY.0b013e3180f62357. [DOI] [PubMed] [Google Scholar]
- 3.Hu YR, Goldman N. Mortality differentials by marital status—an international comparison. Demography. 1990;27(2):233–250. [PubMed] [Google Scholar]
- 4.Hughes ME, Waite LJ. Health in household context: living arrangements and health in late middle age. J Health Soc Behav. 2002;43(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Huijts T, Kraaykamp G. Marital status, nation marital status composition, and self assessed health: a multilevel test of four hypotheses in 29 European countries. Eur Soc. 2011;13(2):279–305. [Google Scholar]
- 6.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the National Longitudinal Mortality Study. Ann Epidemiol. 2000;10(4):224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 7.Kisker EE, Goldman N. Perils of single life and benefits of marriage. Soc Biol. 1987;34(3-4):135–152. doi: 10.1080/19485565.1987.9988671. [DOI] [PubMed] [Google Scholar]
- 8.Koskenvuo M, Kaprio J, Kesaniemi A, Sarna S. Differences in mortality from ischemic heart disease by marital status and social class. J Chronic Dis. 1980;33(2):95–106. doi: 10.1016/0021-9681(80)90033-8. [DOI] [PubMed] [Google Scholar]
- 9.LaHorgue Z. Morbidity and marital status. J Chronic Dis. 1960;12(4):476–498. doi: 10.1016/0021-9681(60)90072-2. [DOI] [PubMed] [Google Scholar]
- 10.Martikainen P, Martelin T, Nihtila E, Majamaa K, Koskinen S. Differences in mortality by marital status in Finland from 1976 to 2000: analyses of changes in marital-status distributions, socio-demographic and household composition, and cause of death. Popul Stud (Camb) 2005;59(1):99–115. doi: 10.1080/0032472052000332737. [DOI] [PubMed] [Google Scholar]
- 11.Martikainen P, Valkonen T. Mortality after the death of a spouse: rates and causes of death in a large Finnish cohort. Am J Public Health. 1996;86(8 pt 1):1087–1093. doi: 10.2105/ajph.86.8_pt_1.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy M, Glaser K, Grundy E. Marital status and long-term illness in Great Britain. J Marriage Fam. 1997;59(1):156–164. [Google Scholar]
- 13.Murphy M, Grundy E, Kalogirou S. The increase in marital status differences in mortality up to the oldest age in seven European countries, 1990–99. Popul Stud (Camb) 2007;61(3):287–298. doi: 10.1080/00324720701524466. [DOI] [PubMed] [Google Scholar]
- 14.Murphy M, Vessey M, Villard L. Marital status and cervical cancer in young women. Lancet. 1989;333(8651):1385–1386. doi: 10.1016/s0140-6736(89)92826-2. [DOI] [PubMed] [Google Scholar]
- 15.Robards J, Evandrou M, Falkingham J, Vlachantoni A. Marital status, health and mortality. Maturitas. 2012;73(4):295–299. doi: 10.1016/j.maturitas.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenborn CA. Marital status and health: United States, 1999–2002. Adv Data. 2004;2004(351):1–32. [PubMed] [Google Scholar]
- 17.Verbrugge LM. Marital status and health. J Marriage Fam. 1979;41(2):267–285. [Google Scholar]
- 18.Zheng H, Thomas PA. Marital status, self-rated health, and mortality: overestimation of health or diminishing protection of marriage? J Health Soc Behav. 2013;54(1):128–143. doi: 10.1177/0022146512470564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Grundy EM, Tomassini C. Marital history, health and mortality among older men and women in England and Wales. BMC Public Health. 2010;10:554. doi: 10.1186/1471-2458-10-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser K, Murphy M, Grundy E. Limiting long-term illness and household structure among people aged 45 and over, Great Britain 1991. Ageing Soc. 1997;17(1):3–19. [Google Scholar]
- 22.Office for National Statistics, UK. Marriage and cohabitation. Table title: has there been an increase in the cohabiting population? 2014. Available at: http://www.ons.gov.uk/ons/rel/census/2011-census-analysis/how-have-living-arrangements-and-marital-status-in-england-and-wales-changed-since-2001-/sty-5-facts.html. Accessed January 1, 2015.
- 23.Kravdal O, Grundy E, Lyngstad TH, Wiik KA. Family life history and late mid-life mortality in Norway. Popul Dev Rev. 2012;38(2):237–257. [Google Scholar]
- 24.Loucks EB, Berkman LF, Gruenewald TL, Seeman TE. Social integration is associated with fibrinogen concentration in elderly men. Psychosom Med. 2005;67(3):353–358. doi: 10.1097/01.psy.0000160482.89163.e8. [DOI] [PubMed] [Google Scholar]
- 25.Holt-Lunstad J, Birmingham W, Jones BQ. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann Behav Med. 2008;35(2):239–244. doi: 10.1007/s12160-008-9018-y. [DOI] [PubMed] [Google Scholar]
- 26.Power C, Elliott J. Cohort profile: 1958 British Birth Cohort (National Child Development Study) Int J Epidemiol. 2006;35(1):34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 27.Danesh J, Lewington S, Thompson SG, Lowe GDO, Collins R Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality—an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 28.Danesh J, Whincup P, Walker M et al. Fibrin d-dimer and coronary heart disease—prospective study and meta-analysis. Circulation. 2001;103(19):2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 29.Tabassum F, Kumari M, Rumley A, Lowe G, Power C, Strachan DP. Effects of socioeconomic position on inflammatory and hemostatic markers: a life-course analysis in the 1958 British Birth Cohort. Am J Epidemiol. 2008;167(11):1332–1341. doi: 10.1093/aje/kwn055. [DOI] [PubMed] [Google Scholar]
- 30.Calvin CM, Batty GD, Lowe GD, Deary IJ. Childhood intelligence and midlife inflammatory and hemostatic biomarkers: the National Child Development Study (1958) cohort. Health Psychol. 2011;30(6):710–718. doi: 10.1037/a0023940. [DOI] [PubMed] [Google Scholar]
- 31.Hyppönen E, Berry D, Cortina-Borja M, Power C. 25-hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: a cross sectional analysis in the 1958 British Birth Cohort. PLoS ONE. 2010;5(5):e10801. doi: 10.1371/journal.pone.0010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demey D, Berrington A, Evandrou M, Falkingham J. Pathways into living alone in mid-life: diversity and policy implications. Adv Life Course Res. 2013;18(3):161–174. doi: 10.1016/j.alcr.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Nagin DS, Tremblay RE. What has been learned from group-based trajectory modeling? Examples from physical aggression and other problem behaviors. Ann Am Acad Pol Soc Sci. 2005;602(1):82–117. [Google Scholar]
- 34.Colman I, Ploubidis GB, Wadsworth MEJ, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Biol Psychiatry. 2007;62(11):1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Mavandadi S, Rook KS, Newsom JT. Positive and negative social exchanges and disability in later life: an investigation of trajectories of change. J Gerontol B Psychol Sci Soc Sci. 2007;62(6):S361–S370. doi: 10.1093/geronb/62.6.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturgis P, Sullivan L. Exploring social mobility with latent trajectory groups. J R Stat Soc Ser A Stat Soc. 2008;171(1):65–88. [Google Scholar]
- 37.Enders CK. A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling. 2001;8(1):128–141. [Google Scholar]
- 38.Little RJA, Rubin DB. The analysis of social science data with missing values. Sociol Methods Res. 1989;18(2–3):292–326. [Google Scholar]
- 39.Little RJA, Rubin DB. Statistical Analysis With Missing Data. second ed. Chichester, UK: Wiley; 2002. [Google Scholar]
- 40.Noah Uhrig S. The Nature and Causes of Attrition in the British Household Panel Survey. Colchester, UK: Institute for Social and Economic Research; 2008. [Google Scholar]
- 41.Durrant G, Goldstein H. Analysing the Probability of Attrition in a Longitudinal Survey. Southampton, UK: Southampton Statistical Sciences Research Institute; 2008. [Google Scholar]
- 42.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh ed. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 43.Wu Z, Hart R. The effects of marital and nonmarital union transition on health. J Marriage Fam. 2002;64(2):420–432. [Google Scholar]
- 44.Waldron I, Hughes ME, Brooks TL. Marriage protection and marriage selection—prospective evidence for reciprocal effects of marital status and health. Soc Sci Med. 1996;43(1):113–123. doi: 10.1016/0277-9536(95)00347-9. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Umberson DJ. The times they are a changin’: marital status and health differentials from 1972 to 2003. J Health Soc Behav. 2008;49(3):239–253. doi: 10.1177/002214650804900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lillard LA, Waite LJ. ’Til death do us part—marital disruption and mortality. Am J Sociol. 1995;100(5):1131–1156. [Google Scholar]
- 47.Pollard M, Mullan Harris K. Nonmarital Cohabitation, Marriage, and Health Among Adolescents and Young Adults. Working paper WR-997. Santa Monica, CA: RAND; 2013.
- 48.Brown SL, Bulanda JR, Lee GR. The significance of nonmarital cohabitation: marital status and mental health benefits among middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60(1):S21–S29. doi: 10.1093/geronb/60.1.s21. [DOI] [PubMed] [Google Scholar]
- 49.Phillips JA, Sweeney MM. Premarital cohabitation and marital disruption among white, black, and Mexican American women. J Marriage Fam. 2005;67(2):296–314. [Google Scholar]
- 50.Trent K, South SJ. Sociodemographic status, parental background, childhood family structure, and attitudes toward family formation. J Marriage Fam. 1992;54(2):427–439. [Google Scholar]
- 51.Trent K, South SJ. Spousal alternatives and marital relations. J Fam Issues. 2003;24(6):787–810. [Google Scholar]
- 52.Williams K, Umberson D. Marital status, marital transitions, and health: a gendered life course perspective. J Health Soc Behav. 2004;45(1):81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joung IMA, Van de Mheen HD, Stronks K, Van Poppel FWA, MacKenbach JP. A longitudinal study of health selection in marital transitions. Soc Sci Med. 1998;46(3):425–435. doi: 10.1016/s0277-9536(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 54.Goldman N. Marriage selection and mortality patterns—inferences and fallacies. Demography. 1993;30(2):189–208. [PubMed] [Google Scholar]
- 55.Yannakoulia M, Panagiotakos D, Pitsavos C, Skoumas Y, Stafanadis C. Eating patterns may mediate the association between marital status, body mass index, and blood cholesterol levels in apparently healthy men and women from the ATTICA study. Soc Sci Med. 2008;66(11):2230–2239. doi: 10.1016/j.socscimed.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 56.Ortega FB, Brown WJ, Lee D-c, Baruth M, Sui X, Blair SN. In fitness and health? A prospective study of changes in marital status and fitness in men and women. Am J Epidemiol. 2011;173(3):337–344. doi: 10.1093/aje/kwq362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martikainen P, Martelin T, Nihtilä E, Majamaa K, Koskinen S. Differences in mortality by marital status in Finland from 1976 to 2000: analyses of changes in marital-status distributions, socio-demographic and household composition, and cause of death. Popul Stud. 2005;59(1):99–115. doi: 10.1080/0032472052000332737. [DOI] [PubMed] [Google Scholar]
- 58.Lindström M. Marital status, social capital, material conditions and self-rated health: a population-based study. Health Policy. 2009;93(2-3):172–179. doi: 10.1016/j.healthpol.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Martikainen P, Valkonen T. Mortality after death of spouse in relation to duration of bereavement in Finland. J Epidemiol Community Health. 1996;50(3):264–268. doi: 10.1136/jech.50.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


