Figure 2.
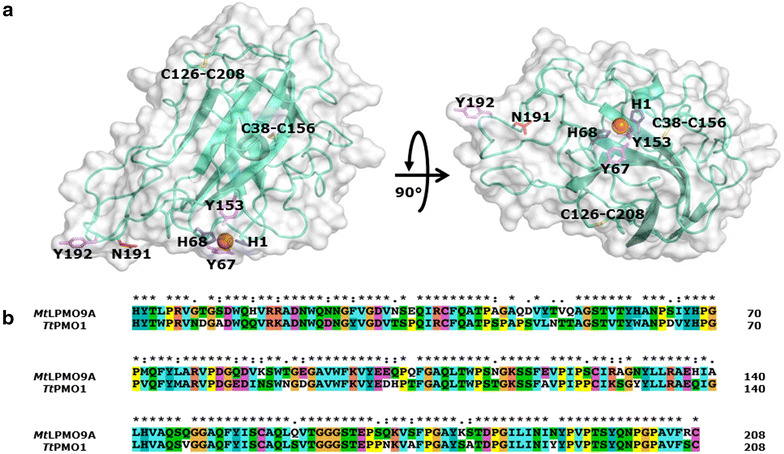
Structural model of MtLPMO9A. a Structural model of MtLPMO9A generated using the available template structure of TtPMO1 from Thielavia terrestris (PDB-id: 3eii) [29]. The divalent metal ion (orange) in the flat face is coordinated by two histidines (His1 and His68; blue) and one tyrosine (Tyr153, magenta), which is typical for LPMOs belonging to subgroup AA9 of the CAZy database [30]. Compared to TtPMO1, Tyr191 is replaced by Asn191 in the flat face. Two disulfide bridges, Cys126–Cys208 and Cys38–Cys156, are conserved and expected to be crucial for the thermotolerance of MtLPMO9A. b Sequence alignment of MtLPMO9A and TtPMO1 (PDB-id: 3eii), which scored the highest in a Blast search using the MtLPMO9A sequence against the Protein Data Bank (75% amino acid identity).
