Abstract
Small nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs) usually exhibit minimal or no growth over many years. However, there is a controversy regarding the optimal management of incidentally discovered, small NF-PNETs. This study aimed to gain insights into tumor behavior and potential strategies for clinical management.
We retrospectively reviewed a total of 202 patients with a suspected PNET (size 2 cm or smaller) at Samsung Medical Center from January 1, 1995 to April 30, 2012. Among these patients, 72 patients were excluded and 145 patients were enrolled in our study. Patients were included if the size of the tumor was ≤2 cm without familial syndrome, radiographic evidence of local invasion or metastases.
Among the 145 patients, 76 patients (52.4%) had pathologically confirmed PNETs. Eleven (14.5%) and 3 (3.9%) of these 76 patients were diagnosed with NET G2 and G3, respectively. PNETs measuring 1.5 cm or more in size had a higher probability of being classified as NET G2 or G3 compared with PNETs measuring <1.5 cm (P = 0.03). Older age (≥55 years) and a meaningful tumor growth (≥20% or ≥5 mm) were significantly associated with NET G2 or G3 (P < 0.05).
Older age (≥55 years), larger tumor size (≥1.5 cm), and a meaningful tumor growth (≥20% or ≥5 mm) were associated with NET G2 or G3. Intensive follow-up could be an acceptable approach in small (especially <1.5 cm), asymptomatic, NF-PNETs.
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) are uncommon neoplasms which originate from diffuse neuroendocrine cells. They have an incidence of approximately 1 in 100,0001 and account for 1% to 2% of all pancreatic tumors.2 Although they may manifest at any age, they most often occur in the sixth to eighth decades of life.3 Most of the PNETs are sporadic; however, some of them can be associated with inherited genetic syndromes such as Multiple Endocrine Neoplasia Type 1 (MEN1) or Von Hippel–Lindau syndrome.
The clinical presentation varies depending on whether the tumor is functioning or nonfunctioning, and which hormones are produced. In recent years, there has been a significant increase in the number of incidentally discovered NF-PNETs because of the widespread use of high-quality cross-sectional imaging and ultrasound, with one of the studies showing a greater than 2-fold increase from 1986 to 2002.4
Surgical resection is the treatment of choice for patients with functional tumors, and it is preferred for most of the NF-PNETs.5–7 It is debatable whether all of the small and asymptomatic lesions should be routinely resected.8 There is insufficient information about the natural history of NF-PNETs, especially when they are small. One of the studies showed that most of the neoplasms ≤2 cm are likely to be benign or intermediate-risk lesions, and only 6% of NF-PNETs ≤2 cm are malignant when incidentally discovered.9 In this setting, a nonoperative approach could be advocated in selected cases for tumors ≤2 cm that are discovered incidentally. Another recent study also suggested that nonoperative management of asymptomatic sporadic NF-PNETs <2 cm is safe in selected patients.10
Therefore, the aim of this study was to gain insights into behavior and potential strategies for clinical management of incidentally discovered small NF-PNETs (2 cm or less).
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board of Samsung Medical Center. We retrospectively reviewed a total of 202 patients with a suspected PNET (size 2 cm or smaller) from January 1, 1995, through April 30, 2012. Patients were identified by keyword search through databases of radiology, pathology, surgery, and gastroenterology. Diagnosis was made by computed tomography (CT) (Brilliance 40; Philips, Eindhoven, Netherlands, USA), magnetic resonance imaging (MRI) (Achieva 3.0T; Philips), or endoscopic ultrasound (EUS) (GF UCT 240; Olympus, Tokyo, Japan) with or without fine-needle aspiration (FNA).
Inclusion criteria included the following: (i) a primary imaging diagnosis of a PNET measuring 2 cm or less in size; (ii) tumors must have been incidentally discovered; and (iii) absence of symptoms suggestive of pancreatic disease (ie, epigastric pain, jaundice, pancreatitis, or symptoms due to hormone hypersecretion). Functioning tumors were identified on the basis of clinical syndromes, and not simply by increased serum hormone levels.
Exclusion criteria were as follows: (i) false-positive preoperative imaging finding of PNETs, but later confirmed to have other tumors, such as accessory spleen or different pancreatic pathologies; (ii) radiographic signs of local invasion, including ductal obstruction, venous thrombosis or narrowing of the vein, invasion of adjacent structures or node or other distant metastases; (iii) inherited genetic syndromes associated with PNETs; and (iv) less than 12 months of follow-up.
Tumor size was recorded by an experienced radiologist using the same method of assessment, based on the Response Evaluation Criteria In Solid Tumors 1.1 (RECIST 1.1) criteria. Tumor growth was compared with the baseline to assess tumor progression. We defined that at least a 20% increase or an absolute increase of at least 5 mm in the tumor diameter as assessed by the imaging study was a meaningful tumor growth. We evaluated the characteristics of NET G1, G2, and G3 in the pathologically confirmed group.
Histological Assessment
All available pathological slides were reviewed and histopathological classification and grades were revised, if necessary, by specialized pathologists. All patients were reclassified according to the 2010 World Health Organization (WHO) classification.11 Tumors were classified as G1 (well-differentiated with benign characteristics) or G2 (well-differentiated with low-grade malignant characteristics), or G3 (poorly differentiated with high-grade malignant characteristics). Neuroendocrine carcinoma (NEC) refers to all poorly differentiated G3 NETs.
Follow-Up
Depending on the morphology and size of the lesions, patients underwent imaging studies at 3-, 6-, and 12-month intervals. Follow-up for the nonoperative group was initiated when the neoplasm was first diagnosed on index imaging. Follow-up for the operative group was initiated at the time of surgical resection. Patients undergoing surgery were regularly followed up after resection. Follow-up evaluation included clinical examination and/or imaging studies such as CT or MRI. It was considered that recurrence had occurred if the imaging studies demonstrated new lesions suspicious for NET in the pancreatic remnant. Patients who had incomplete records were contacted by mail and/or telephone, and their most recent imaging study was obtained whenever possible. Follow-up data were collected until May 1, 2013.
Statistical Analysis
Descriptive statistics were reported as either frequencies (percentages) or means (ranges) as appropriate. Categorical variables were compared using the Pearson's chi-square or Fisher's exact test. Continuous variables were compared by the Mann–Whitney U test, or, if they had a normal distribution, by using the 2-sample Student t test. Independent factors influencing the efficacy were evaluated by a univariate analysis and confirmed by a logistic regression (Backward, likelihood ratios). Receiver operating characteristics (ROC) analysis was performed to evaluate the cut-off value of tumor size. P values of <0.05 were considered statistically significant. The SPSS 19 software for Windows (SPSS, Inc, Chicago, IL) was used for all analyses.
RESULTS
A total of 202 patients who had a clinical diagnosis of asymptomatic, NF-PNETs (size 2 cm or smaller) during the study period were identified. Among these patients, 72 patients (49.7%) underwent surgical resection (60 patients at the time of diagnosis and 12 patients after follow-up) and 73 patients were managed by an ongoing follow-up. These 145 patients were enrolled in our study, and 57 patients were excluded for the following reasons; 13 patients confirmed to have other tumors (accessory spleen, 5 patients; solid pseudopapillary tumor, 4 patients; serous cystadenoma, 3 patients; pancreatic ductal adenocarcinoma, 1 patient), 14 patients with local invasion or distant metastasis, 6 patients diagnosed with MEN1 or Von Hippel–Lindau syndrome, and 24 patients followed for <1 year. Finally, 76 patients (52.4%) had pathologically confirmed PNETs (Figure 1).
FIGURE 1.
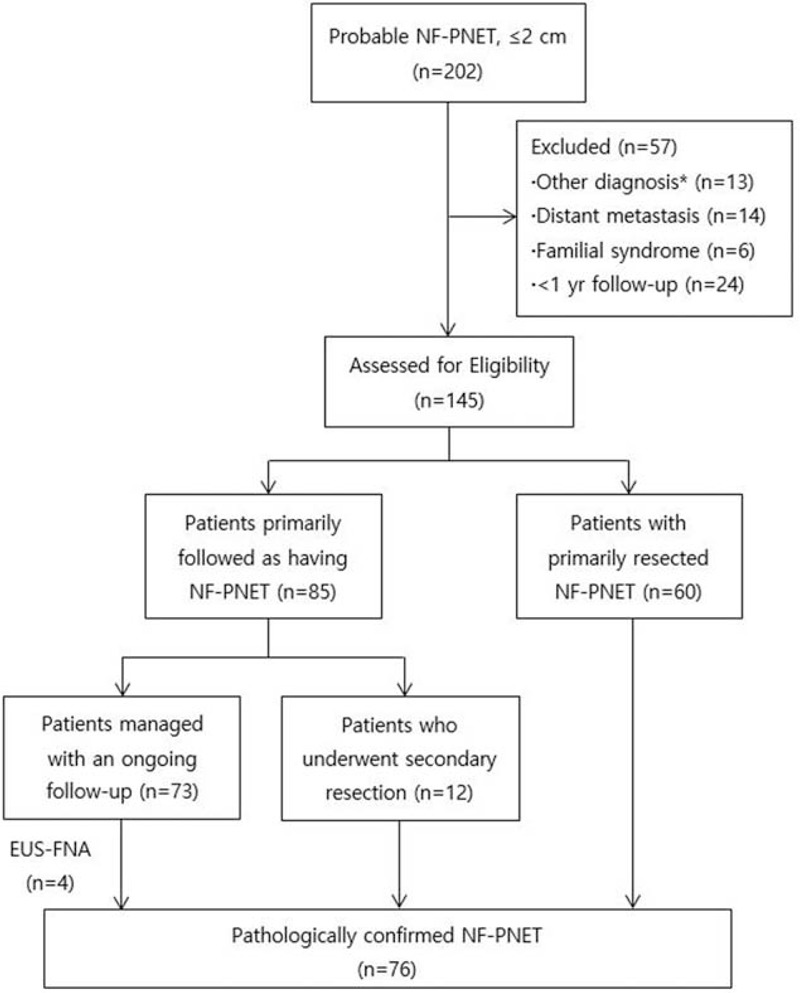
Flowchart and management of 202 patients with NF-PNETs (nonfunctioning pancreatic neuroendocrine tumors). ∗Accessory spleen, 5 patients; solid pseudopapillary tumor, 4 patients; serous cystadenoma, 3 patients; pancreatic ductal adenocarcinoma, 1 patient.
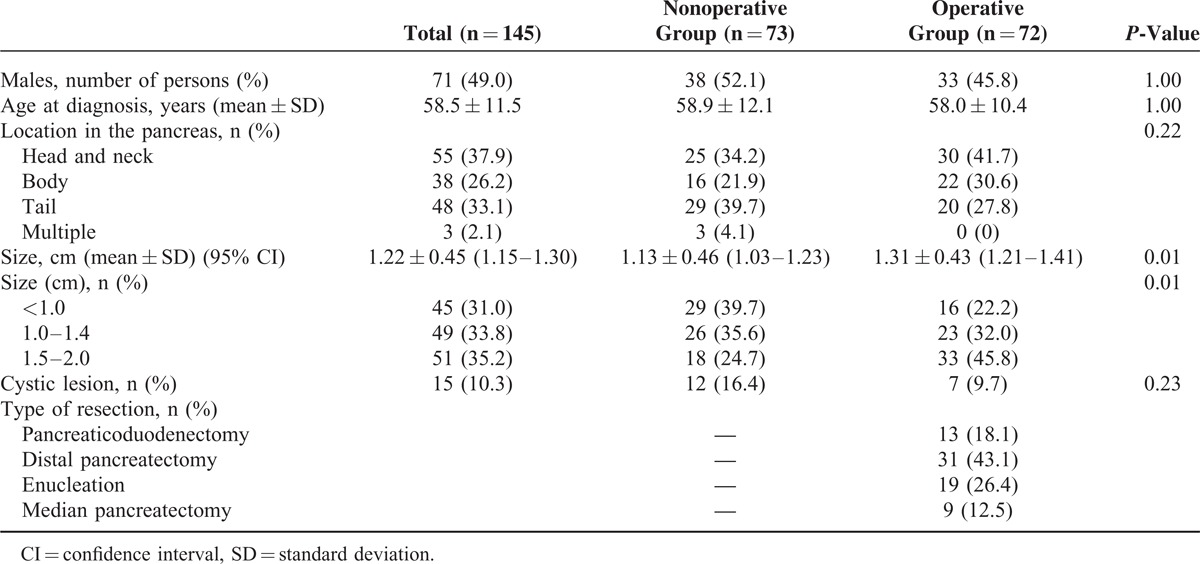
The baseline characteristics of patients are shown in Table 1. The patients comprised 71 men and 74 women (mean age, 58.5 years). Tumors were most commonly found in the head and neck of the pancreas (n = 55, 37.9%). Patients undergoing resection (operative group) had a larger tumor size than those managed by observation (nonoperative group) (1.31 cm vs. 1.13 cm; P = 0.01). Patients having a tumor size of 1.5 to 2.0 cm were more likely to undergo surgical resection (33 vs. 18; P = 0.01) (Table 1).
TABLE 1.
Baseline Characteristics of the Patients
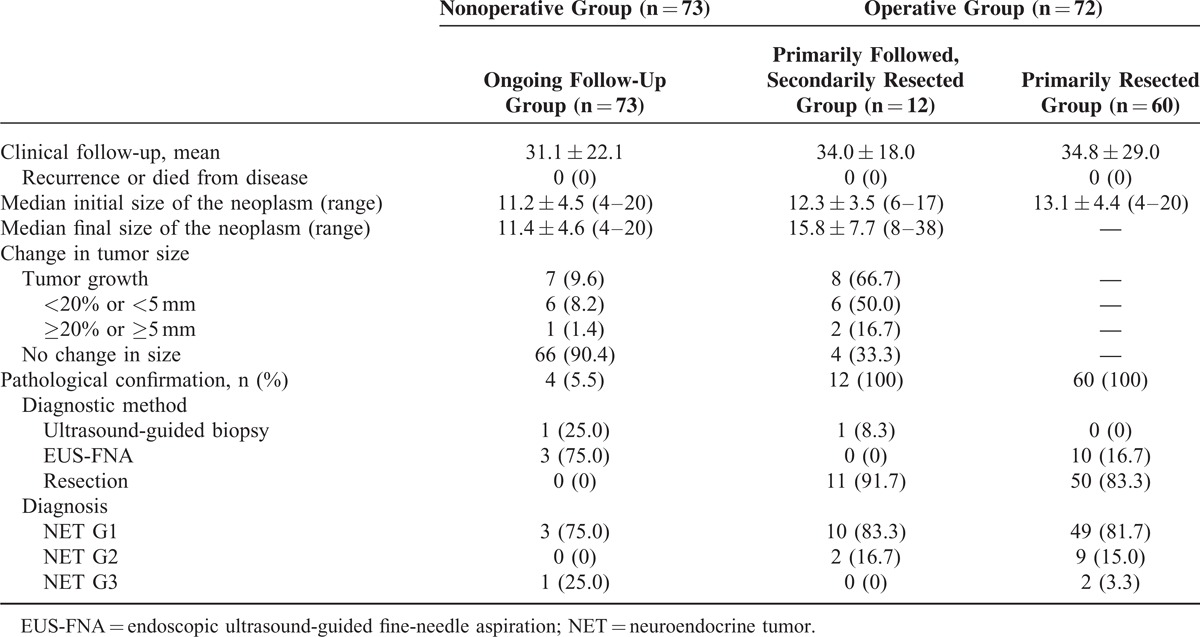
In the primarily followed group (combining the ongoing follow-up group and the primarily followed, secondarily resected group; n = 85) (Figure 1), 70 patients (82.4%) had no change in tumor size and 12 patients (14.1%) had a minimal increase in tumor size (<20% or <5 mm). However, 3 patients (3.5%) had a meaningful tumor growth (≥20% or ≥5 mm). In the ongoing follow-up group, 7 patients (9.6%) had an increase in tumor size. Among these 7 patients, only 1 patient had a meaningful tumor growth. Sixty-six patients (90.4%) had no change in tumor size during the follow-up. In the primarily followed, secondarily resected group, 8 patients underwent surgery because of increase in tumor size, 1 patient developed symptoms, and 3 patients opted for surgery during follow-up. Among them, 2 patients (16.7%) had a meaningful tumor growth. There were no cases of disease-related death or recurrence during the clinical follow-up in all of the groups (Table 2).
TABLE 2.
Comparison of the Baseline Characteristics in the Nonoperative Group and Operative Group
Finally, 76 patients were pathologically confirmed: 62 patients (81.6%) had NET G1, 11 patients (14.5%) had NET G2, and 3 patients (3.9%) had NET G3. In the pathologically confirmed group (n = 76), 18.4% of patients were diagnosed with NET G2 or G3. When the tumor size was <1.5 cm, only 9.8% of patients with NF-PNETs were classified as NET G2 and none of the patients were diagnosed with NET G3. PNETs measuring 1.5 cm or more in size had a higher probability of being classified as NET G2 or G3 compared with PNETs measuring less than 1.5 cm (P = 0.03) (Figure 2).
FIGURE 2.
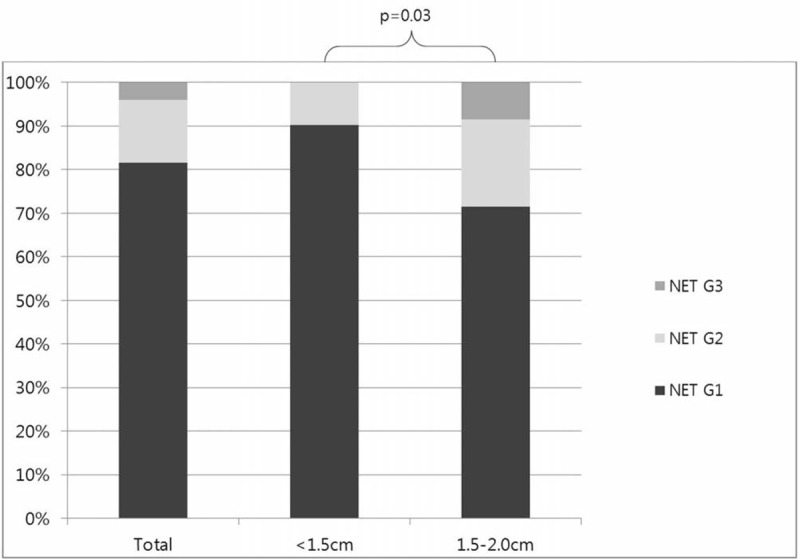
Pathologic results of PNET (pancreatic neuroendocrine tumor) according to the tumor size. NET, neuroendocrine tumor.
A meaningful tumor growth (≥20% or ≥5 mm) was observed in NET G2 or G3 (P < 0.01) (Table 3). The patients who were 55 years or older had a higher probability of having NET G2 or G3 (P = 0.04). The mean follow-up period for NET G1 and NET G2 or G3 was 33 and 45 months, respectively. None of the cases had disease recurrence or died of their disease during the follow-up period (Table 4). To evaluate the clinical factors related to NET G2 or G3, multivariate analyses were performed. The multivariate analysis revealed that a tumor size (1.5 cm or more) was independent factor, predictive of NET G2 or G3, with odds ratios of 3.70 (1.04–13.12) (Table 5).
TABLE 3.
Comparison of the Pathologic Results According to the Variation in Tumor Size in the Pathologically Confirmed Group
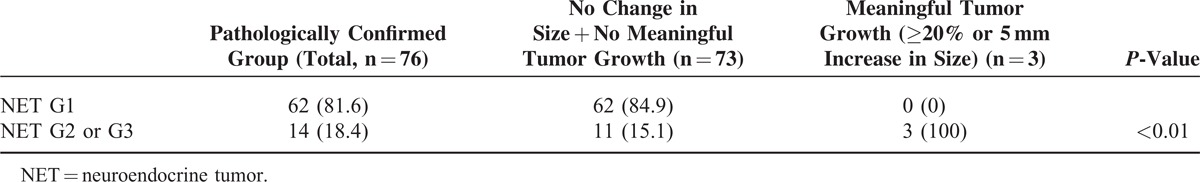
TABLE 4.
Comparison of the Characteristics of Neuroendocrine Tumor (NET) G1 and NET G2 or G3
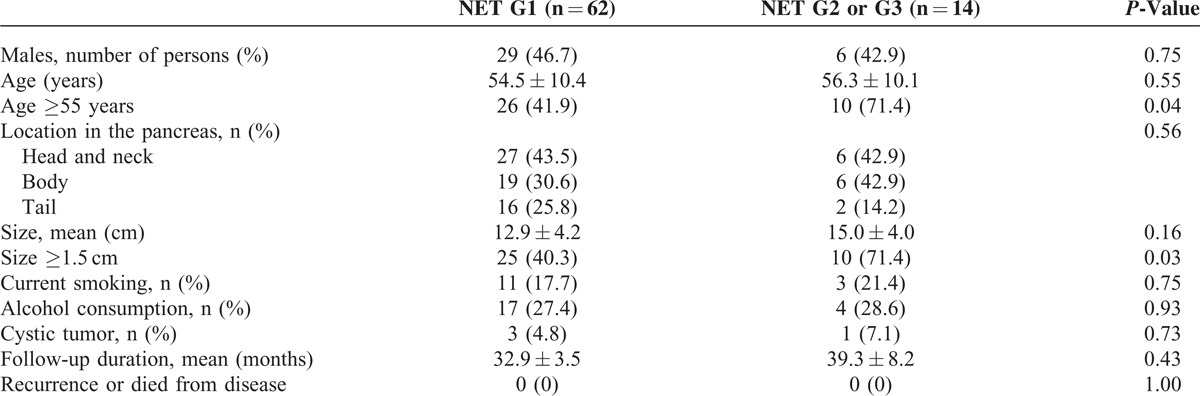
TABLE 5.
Multivariate Analysis for the Clinical Factors Related to the Neuroendocrine Tumor (NET) G2 or G3

DISCUSSION
PNETs are a heterogeneous group of neoplasms with diverse clinical findings. They can be either functioning or NF-PNETs.12 Functional tumors secrete hormones and produce characteristic endocrine syndromes. NF-PNETs can secrete various products (chromogranin A, neuron-specific enolase, pancreatic polypeptide, etc.) but do not cause symptoms.13 Many small NF-PNETs are discovered incidentally on cross-sectional imaging (CT/MRI) which is being performed more frequently nowadays.2,14 Recently, the H. Lee Moffitt Cancer Center concluded that 40% of PNETs were discovered incidentally and 55% of tumors were in stage I according to American Joint Committee on Cancer (AJCC) classification.15
The WHO classification of PNETs was updated in 2010. This grading classification uses the mitotic rate and Ki-67 labeling index.16 General features of prognostic significance include tumor size, vascular invasion, mitotic rate, proliferation index, and nodal and distant metastases.17 According to the WHO classification of NF-PNETs, the tumor size correlates with the potential for malignancy, and localized tumors measuring >2 cm should preferentially be treated with standard pancreatic resections.6
However, the natural history of PNETs is not well-established, and the proper management and follow-up strategy of incidentally discovered small PNETs are controversial. Some authors suggest that for NF-PNETs measuring ≤2 cm, a nonoperative management can be considered, and the risks and benefits of surgical resection should be carefully weighed in patients with small lesions.6,10,18,19 There is accumulating evidence regarding the risks associated with pancreatic surgery of PNETs. An analysis of the Nationwide Inpatient Sample from 1998 to 2006 demonstrated an in-hospital mortality rate of 1.7% and an overall complication rate of 29.6% after pancreatectomy for PNETs.20 The majority of complications involved postoperative infections (11.1%), digestive system complications (8.8%), or pulmonary compromise (7.3%). In-hospital mortality rate was 1.7%. Other authors suggest that surgery is indicated in any case because NF-PNETs should always be considered as potentially malignant tumors, and a proper histological examination of the tumor (including the mitotic and Ki-67 indexes) is possible only on the resected specimen.21,22
Previous studies have reported that several factors are associated with survival, including age,23,24 tumor size,9,23,25–27 grade/differentiation,14,24,25,28 LN status,25,29 presence of distant metastases,14,23,24,29 and surgical resection.14,23 Age has consistently been found to be a powerful predictor of survival in patients undergoing resection of PNETs.30 Similarly, the present study demonstrated that older age (≥55 years) was associated with NET G2 or G3.
Most of the neoplasms measuring ≤2 cm are likely to be benign or intermediate-risk lesions. When incidentally discovered small tumors were considered, only 6% of the NF-PNETs measuring ≤2 cm were malignant and none of the patients died of the disease.9 In the present study, none of the NF-PNETs (<1.5 cm) and only 3.9% of NF-PNETs (≤2 cm) that were discovered incidentally and confirmed pathologically were classified as NET G3. Interestingly, only 1 patient in our study who was observed by an ongoing follow-up was diagnosed as having NET G3. Because this patient also had nonsmall cell lung cancer, NET G3 was discovered incidentally during cancer work-up and additional treatment was not performed. There were no cases of disease-related death or recurrence during the clinical follow-up in our study. In the ongoing follow-up group, most of the patients were safely observed without any clinical problems.
Therefore, a nonoperative approach could be advocated for NF-PNETs measuring ≤2 cm (especially <1.5 cm) that are discovered incidentally. This more conservative approach would be the most suitable for higher-risk patients with significant medical comorbidities. An intensive 3-month follow-up for the first year and then a 6-month follow-up up to 3 years could be recommended in these patients. The present study showed that a meaningful tumor growth was associated with more malignant lesions (NET G2 or G3). If a meaningful increase in the tumor size is detected during the follow-up, prompt operative resection should be considered.9
Diagnosis remains uncertain without confirmation by biopsy or resection specimen analysis. Only 5.5% of patients in the ongoing follow-up group had confirmation regarding the diagnosis of a NF-PNET by a biopsy, which raises the question of the accuracy of the diagnosis in the remaining patients. Despite high-quality imaging of the pancreas, radiologists cannot always distinguish between different pancreatic pathologies or accessory splenic tissue. One of the studies reported that 17% of patients in the nonbiopsy-confirmed, operative NF-PNETs group had false-positive preoperative imaging findings, and one might expect a similar percentage of patients in the nonoperative group.26 Similarly, in our study, 13 patients (18%) in the nonbiopsy-confirmed, operative group had false-positive preoperative imaging finding of NF-PNETs, but later confirmed to have other tumors. Additional preoperative studies such as somatostatin receptor imaging or EUS might improve diagnostic accuracy.
This study has several limitations. First, there is a possibility that patients in the ongoing follow-up group did not have NF-PNETs. The reason for this possibility is the difficulty in distinguishing NF-PNETS from other pancreatic lesions. We included patients with a high probability of being diagnosed as having NF-PNETs. Second, our conclusions are limited by the retrospective nature of the data. A randomized trial comparing between the observation and resection groups would provide more definitive results, but it would be difficult to perform such a study due to the low incidence of NF-PNETs.
In conclusion, older age (≥55 years), larger tumor size (≥1.5 cm) and a meaningful tumor growth (≥20% or ≥5 mm) during follow-up were associated with NET G2 or G3. Especially, larger tumor size (≥1.5 cm) is a significant independent risk factor for NET G2 or G3. Small NF-PNETs usually exhibit minimal or no growth over many years. The choice of the appropriate management for these small tumors should be well-balanced after considering the short- and long-term sequelae following pancreatic resection procedures. Therefore, an intensive follow-up could be an acceptable approach in small (especially <1.5 cm in size), asymptomatic NF-PNETs.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, CT = computed tomography, EUS = endoscopic ultrasound, EUS-FNA = endoscopic ultrasound-guided fine-needle aspiration, MEN1 = Multiple Endocrine Neoplasia Type 1, MRI = magnetic resonance imaging, NEC = neuroendocrine carcinoma, NET = neuroendocrine tumors, NF-PNETs = nonfunctioning pancreatic neuroendocrine tumors, PNETs = pancreatic neuroendocrine tumors, RECIST = the Response Evaluation Criteria In Solid Tumors, ROC = receiver operating characteristics, SD = standard deviation, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010; 45:234–243. [DOI] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008; 19:1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou C, Zhang J, Zheng Y, et al. Pancreatic neuroendocrine tumors: a comprehensive review. Int J Cancer 2012; 131:1013–1022. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald TL, Hickner ZJ, Schmitz M, et al. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas 2008; 37:134–138. [DOI] [PubMed] [Google Scholar]
- 5.Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012; 95:98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012; 95:120–134. [DOI] [PubMed] [Google Scholar]
- 7.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010; 39:735–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Rosa S, Klersy C, Uccella S, et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol 2009; 40:30–40. [DOI] [PubMed] [Google Scholar]
- 9.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011; 150:75–82. [DOI] [PubMed] [Google Scholar]
- 10.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2013; 98:4784–4789. [DOI] [PubMed] [Google Scholar]
- 11.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010; 39:707–712. [DOI] [PubMed] [Google Scholar]
- 12.Dixon E, Pasieka JL. Functioning and nonfunctioning neuroendocrine tumors of the pancreas. Curr Opin Oncol 2007; 19:30–35. [DOI] [PubMed] [Google Scholar]
- 13.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008; 135:1469–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg 2010; 14:541–548. [DOI] [PubMed] [Google Scholar]
- 15.Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol 2012; 19:2932–2936. [DOI] [PubMed] [Google Scholar]
- 16.Rindi G, Kloppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007; 451:757–762. [DOI] [PubMed] [Google Scholar]
- 17.Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol 2008; 19:903–908. [DOI] [PubMed] [Google Scholar]
- 18.Falconi M, Plockinger U, Kwekkeboom DJ, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology 2006; 84:196–211. [DOI] [PubMed] [Google Scholar]
- 19.Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery 2001; 130:1078–1085. [DOI] [PubMed] [Google Scholar]
- 20.Smith JK, Ng SC, Hill JS, et al. Complications after pancreatectomy for neuroendocrine tumors: a national study. J Surg Res 2010; 163:63–68. [DOI] [PubMed] [Google Scholar]
- 21.Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008; 113:256–265. [DOI] [PubMed] [Google Scholar]
- 22.Vilar E, Salazar R, Perez-Garcia J, et al. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 2007; 14:221–232. [DOI] [PubMed] [Google Scholar]
- 23.Martin RC, Kooby DA, Weber SM, et al. Analysis of 6,747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastroint Surg 2011; 15:175–183. [DOI] [PubMed] [Google Scholar]
- 24.Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008; 247:490–500. [DOI] [PubMed] [Google Scholar]
- 25.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011; 146:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee LC, Grant CS, Salomao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery 2012; 152:965–974. [DOI] [PubMed] [Google Scholar]
- 27.Toste PA, Kadera BE, Tatishchev SF, et al. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg 2013; 17:2105–2113. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Choi DW, Choi SH, et al. Surgical strategies for non-functioning pancreatic neuroendocrine tumours. Br J Surg 2012; 99:1562–1568. [DOI] [PubMed] [Google Scholar]
- 29.Krampitz GW, Norton JA, Poultsides GA, et al. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Surg 2012; 147:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherenfant J, Stocker SJ, Gage MK, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 2013; 154:785–791.discussion 791–783. [DOI] [PubMed] [Google Scholar]


