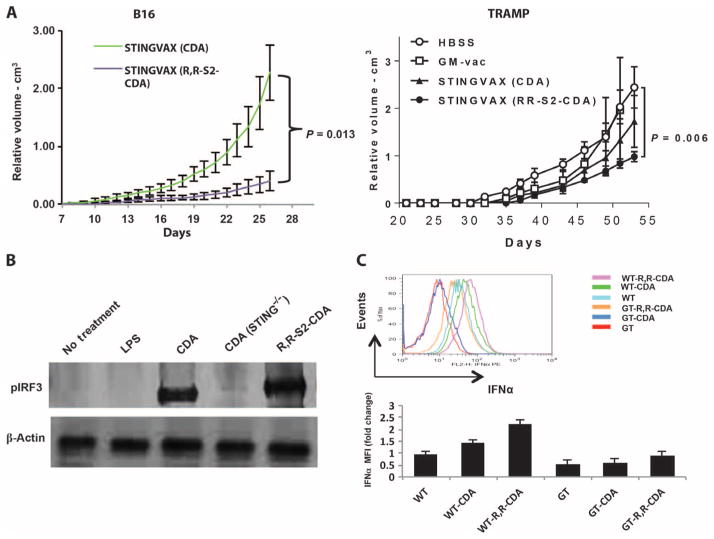
Fig. 5. Synthetic RR-S2 CDA increased STINGVAX’s potency.
(A) STINGVAX was formulated with either canonical CDA or RR-S2 CDA, with equimolar CDN amounts per mouse (20 μg per vaccine), and used to treat palpable B16 and TRAMP tumors. To improve the sensitivity of the in vivo treatment assay between the different STINGVAX formulations, we increased the initial tumor burden for the B16 tumor to 105 cells per inoculation for these experiments. Each group had 10 mice, and data represent means ± SEM. Each graph is representative of three to five experiments. (B) WT and goldenticket (STING−/−) bone marrow–derived murine DCs were incubated with CDA, and the cell lysates were probed with pIRF3 antibody. (C) DCs harvested from lymph nodes of WT and goldenticket (GT) mice were incubated with CDNs overnight, and their IFNα levels were quantitated by flow cytometry. Shown are the data for CD11c+B220+-gated DCs and the MFI change relative to untreated WT DC controls (WT). Bottom panel illustrates the IFNα MFI change relative to WT untreated DC (WT), and the top panel is a representative histogram. Three replicates were performed, and data represent means ± SEM.