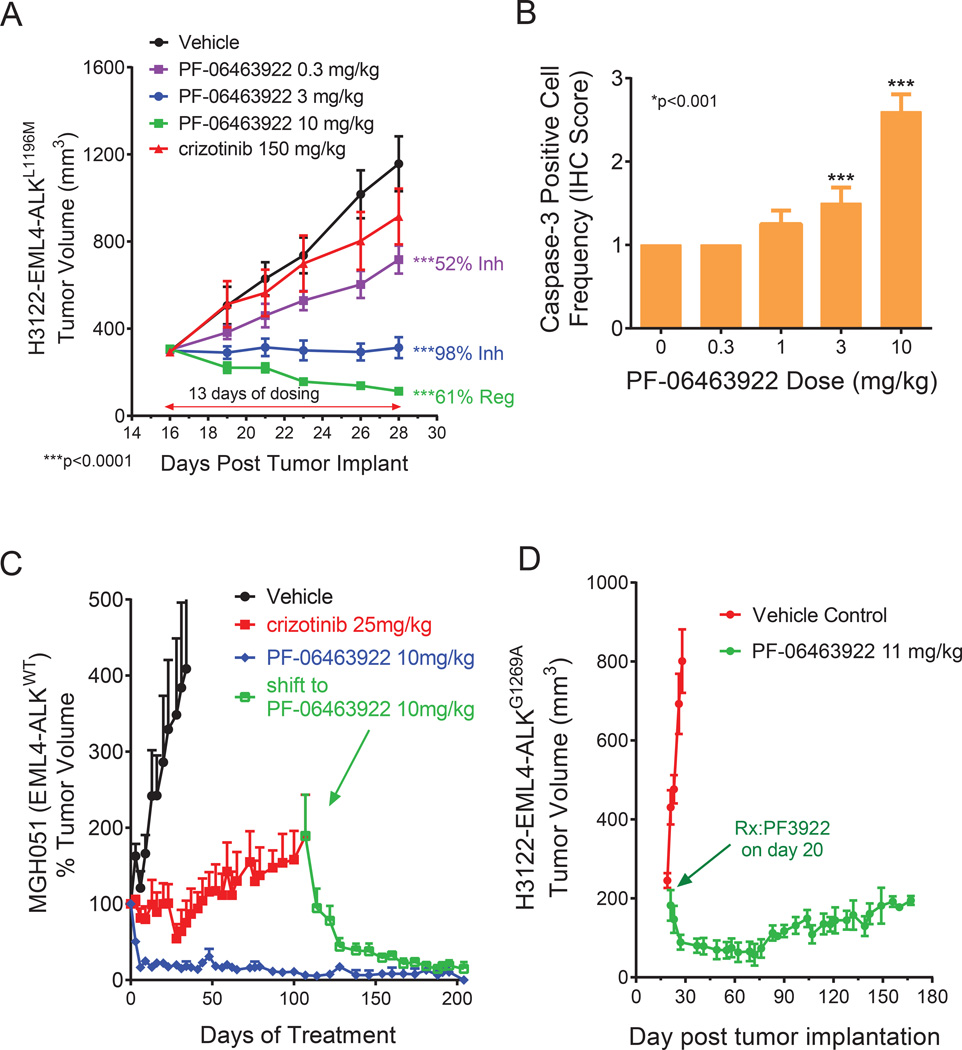
Figure 4. PF-06463922 antitumor efficacy in ALK fusion driven subcutaneous xenograft tumor models in mice.
A) Subcutaneous tumor growth in the H3122 EML4-ALKL1196M tumor model treated with orally dosed crizotinib 75 mg/kg BID or PF-06463922 0.3–10 mg/kg BID for 13 days. Tumor volumes are presented as mean +/− SEM (n=12). B) Activated-Caspase3 positive cell numbers following 3-day of oral BID administration of PF-06463922 in the H3122-EML4-ALKL1196M model (cf. Fig. 4A). Values = Mean +/− SEM (n=7–9). C) Long term subcutaneous tumor growth in the EML4-ALKWT MGH051 crizotinib-resistant patient-derived model treated with crizotinib 25 mg/kg QD or PF-06463922 10 mg/kg BID. The mice treated with crizotinib were shifted to PF-06463922 after 107 days of treatment (arrow). Tumor volumes are presented as mean +/− SD (n=5–12). D) Long term subcutaneous tumor growth of the H3122-EML4-ALKGG1269A tumors treated with PF-06463922 subcutaneous pump infusion at 11 mg/kg/day for 172 days. Tumor volumes are presented as mean +/− SEM (n=5–12). See also Figure S4.