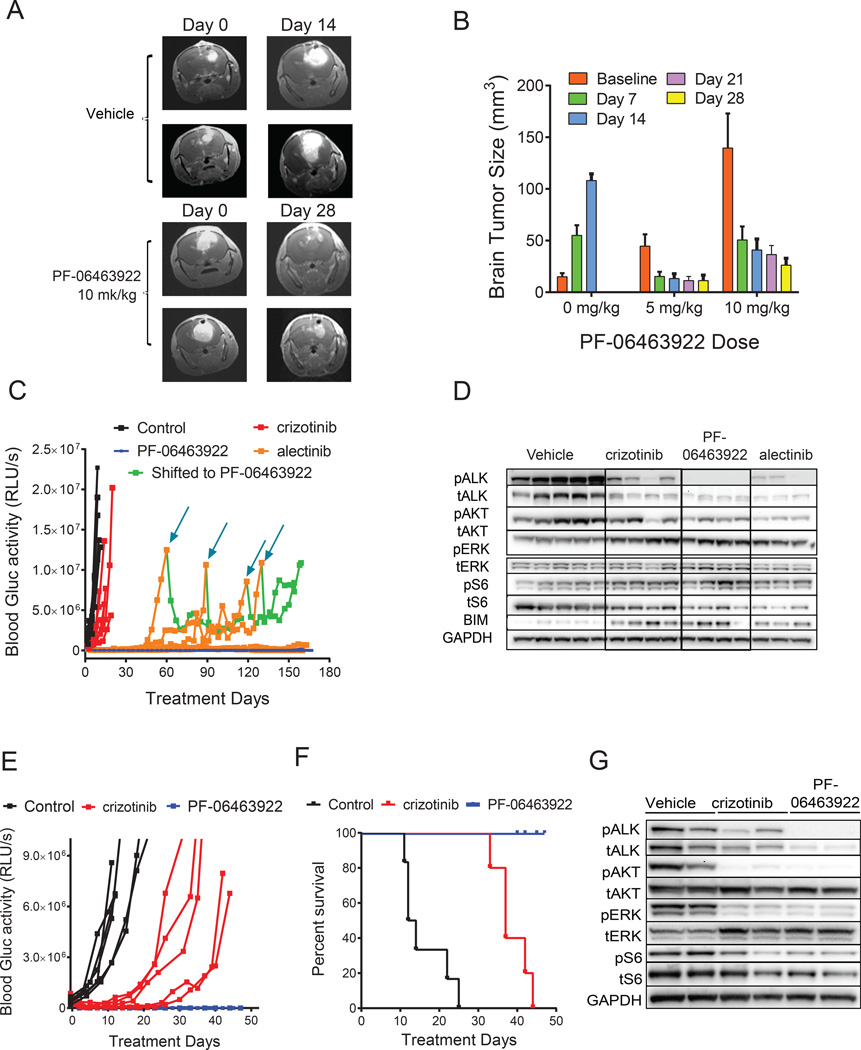
Figure 5. PF-06463922 antitumor efficacy in ALK fusion-driven intracranial tumor models.
A) Representative MRI images showing regression of large established H3122 EML4-ALKWT intracranial tumors in mice following PF-06463922 infusion. B) Quantitation of brain tumor sizes following PF-06463922 treatment in the H3122 EML4-ALKWT intracranial model shown in panel A. Values are presented as mean +/− SEM. C) Oral dosing of PF-06463922, crizotinib and alectinib. Long term brain orthotopic tumor growth of H3122 EML4-ALKWT cells expressing secreted luciferase treated with crizotinib 50 mg/kg QD or alectinib 60 mg/kg QD or PF-06463922 10 mg/kg BID. The mice treated with alectinib were shifted to PF-06463922 at the indicated times (blue arrows). D) Pharmacodynamic analysis of H3122 EML4-ALKWT brain tumors treated for 3 days and collected 3 hours after last treatment. (E,F) Brain tumor growth (E) and Kaplan-Meier survival curves (p<0.0001) (F) of MGH006 EML4-ALKWT patient-derived cell line in mice treated orally dosed with crizotinib 100 mg/kg QD or PF-06463922 10 mg/kg/day BID for 42 days. G) Pharmacodynamic analysis of MGH006 brain tumors treated for 3 days and collected 3 hours after last treatment. Individual blood Gluc activity values are presented for each mouse (n=5–7 per group). See also Figure S5.