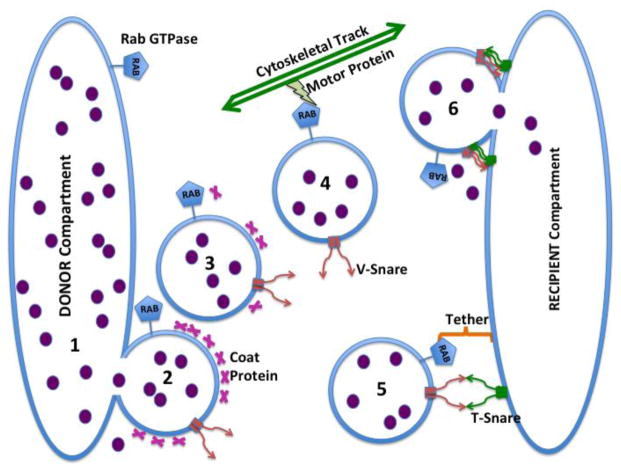
Figure 2. Proposed Mechanism of Vesicular Trafficking of Oligonucleotides.
Oligonucleotides (depicted as small filled circles) are initially accumulated in an endomembrane compartment (the DONOR compartment, for example, early endosomes) and are then trafficked by means of shuttle vesicles to various other endomembrane compartments (the RECIPIENT compartment, for example, the trans-Golgi). The first step (1) involves disjunction (’pinching off’) of a shuttle vesicle under the influence of a coat protein as well as other accessory proteins. At this stage there are non-bilayer regions at the junction between the membranes of the DONOR compartment and the shuttle vesicle. This provides an opportunity for some oligonucleotide to escape to the cytosol. Step 2 involves uncoating of the coated vesicle; Rab proteins can contribute to this step. Step 3 comprises movement of the shuttle vesicle toward its destination along cytoskeletal tracks. Motor proteins such as various myosins (for the actin system) or dyneins or kinesins (for the microtubular system) propel the vesicle. Rab proteins are involved in forming the appropriate linkages to the cytoskeleton. Step 4 entails recognition of the RECIPIENT (‘target’) compartment by the shuttle vesicle. Tether proteins work with Rab proteins to provide interaction specificity while v-SNARE proteins in the vesicle membrane interact with t-SNARE proteins in the RECIPIENT compartment membrane to provide firm bridging, as well as contributing to specificity. In step 5 the SNARE proteins undergo major conformational changes, and with the assistance of accessory proteins, trigger fusion of the shuttle vesicle membrane with the membrane of the RECIPIENT compartment. At this stage non-bilayer regions exist at the junction between shuttle and RECIPIENT membranes potentially allowing escape of oligonucleotide.