Abstract
Introduction
Anticoagulation, fibrinogen consumption, fibrinolytic activation, and platelet dysfunction all interact to produce different clot formation responses after trauma. However, the relative contributions of these coagulation components to overall clot formation remains poorly defined. We examined for sources of heterogeneity in clot formation responses after trauma.
Methods
Blood was sampled in the Emergency Department from patients meeting trauma team activation criteria at an urban trauma center. Plasma prothrombin time (PT) ≥ 18 sec was used to define traumatic coagulopathy. Mean kaolin-activated thrombelastography (TEG) parameters were calculated and tested for heterogeneity using Analysis of Means (ANOM). Discriminant analysis and forward stepwise variable selection with linear regression were used to determine if PT, fibrinogen, platelet contractile force (PCF), and D-Dimer concentration, representing key mechanistic components of coagulopathy, each contribute to heterogeneous TEG responses after trauma.
Results
Of 95 subjects, 16% met criteria for coagulopathy. Coagulopathic subjects were more severely injured with greater shock, and received more blood products in the first 8 hours compared to non-coagulopathic subjects. Mean (SD) TEG maximal amplitude (MA) was significantly decreased in the coagulopathic group=57.5 (4.7) mm, vs. 62.7 (4.7), T test p<0.001. The MA also exceeded the ANOM predicted upper decision limit for the non-coagulopathic group and the lower decision limit for the coagulopathic group at alpha=0.05, suggesting significant heterogeneity from the overall cohort mean. Fibrinogen and PCF best discriminated TEG MA using discriminant analysis. Fibrinogen, PCF, and D-Dimer were primary covariates for TEG MA using regression analysis.
Conclusion
Heterogeneity in TEG-based clot formation in Emergency Department trauma patients was linked to changes in MA. Individual parameters representing fibrin polymerization, platelet contractile forces, and fibrinolysis were primarily associated with TEG MA after trauma and should be the focus of early hemostatic therapies.
INTRODUCTION
Approximately 40% to 70% of potentially salvageable trauma deaths are caused by exsanguination from uncontrollable truncal hemorrhage within the first hours after injury and prior to arrival at medical facilities (1-3). The Primary coagulopathy of trauma (PCoT) includes all biological mechanisms of coagulopathy arising after traumatic injury and is independent from the effects of environmental hypothermia and dilution from fluid resuscitation (4). Hypocoagulability after trauma is one component of PCoT and is an acute acquired coagulation disorder that is immediately present in 25% of injured patients at hospital arrival, carries a 4-6x increased mortality when present, and is associated with increased incidence of multi-organ failure, intensive care utilization, and need for blood transfusion (5-7).
Hypocoagulability is a multifaceted component of trauma pathophysiology that involves coagulation factor inhibition, platelet dysfunction, fibrinogen consumption, and hyperfibrinolysis. These changes occur more often in those trauma victims having both severe anatomical injury and tissue hypoperfusion from major blood loss (8-9). Hypocoagulability was first measured using plasma tests of the extrinsic and intrinsic coagulation pathways by prothrombin time (PT> 18 sec) and activated partial thromboplastin time (aPTT> 60 sec), respectively. (8) More recently viscoelastic hemostatic assays (VHA's) using extrinsically-activated whole blood thrombelastography (TEG) /rotational thromboelastometry (ROTEM) have demonstrated significant prolongation of clot onset times, a reduction of clot strength, and accelerated clot lysis as important indicators of hypocoagulability and outcomes (10-12).
The multifaceted nature of PCoT makes rapid identification and treatment of specific targets for therapy in the Emergency Department quite difficult. VHA's are composite measures, representing overall clot formation in whole blood, and can be limited in their ability to guide specific therapies in a timely manner. To quickly identify and treat hemostatic deficiencies, further clarification of the behavior of VHA's early after trauma and their relation to specific underlying mechanistic sources of coagulopathy are required.
To address this problem, the main objectives of this study were to; 1.) Identify the primary source of heterogeneity in TEG-based clot formation in coagulopathic Emergency Department trauma patients, and 2.) Determine what components of coagulopathy are most strongly associated with heterogeneous clot formation in this cohort. We tested these objectives by performing a prospective cross-sectional study of blood coagulation and clot formation in trauma patients presenting to an urban Level I trauma center.
METHODS
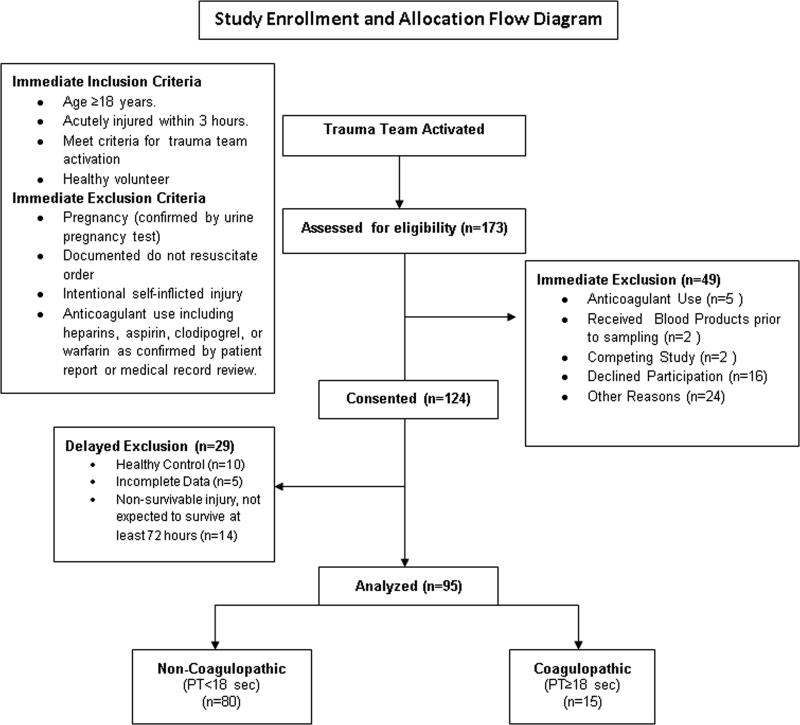
This is a prospective cross-sectional study of trauma patients presenting to an American urban level I trauma center. The study was approved by the institutional human subjects review board. Trauma patients were first identified from trauma team activations. Trauma team activations were activated by the prehospital providers and criteria included severe multisystem injury, high-risk injury mechanism, penetrating injury to the chest or abdomen, abnormal vital signs including hypotension, or requirement for intubation or life-saving maneuver in the field prior to arrival in the Emergency Department. Full trauma alerts included the presence of the attending trauma surgeon in the trauma bay within 15 minutes of patient's arrival to guide resuscitation. Modified trauma alerts were managed by Emergency Medicine attending physicians with assistance from surgical trauma team members. Subjects activated for either full or modified trauma alerts were then screened and enrolled according to Figure 1. Blood was collected into standard citrated vacutainers (1:9 ratio of citrate to blood) prior to receiving any blood products. To avoid confounding influences of blood transfusion, minor injury, or massive non-survivable injury, patients were excluded if they received any blood products prior to study sample blood draw, were not expected to live for 3 days due to obvious non-survivable injury, and/or were minimally-injured and expected to be discharged from the Emergency Department or hospitalized for less than 72 hours. Samples were then processed in a nearby hemostasis laboratory (Virginia Commonwealth University coagulation advancement lab, VCAL, Virginia Commonwealth University School of Pharmacy). Clinical data including vital signs, injury profiles, and outcomes were collected from the medical record and the injury severity scores (ISS) were calculated for each patient. Patients who were found to be pharmacologically anticoagulated by antiplatelet agents (aspirin or clopidogrel) or vitamin K antagonists after chart review were excluded from analysis.
Figure 1.
Study enrollment criteria and allocation.
Measurements of Clot Formation and Coagulation
All measurements of viscoelastic clot formation were made within 2 hours of collection. The viscoelastic properties of whole blood were measured using thrombelastography (TEG 5000, Haemonetics corp. Braintree, MA) using the intrinsic pathway activator kaolin (Kaolin®, Haemonetics corp.) and recalcification to 10mmol/L final calcium concentration. The TEG 5000 reports time to onset of clot formation (R), which positively correlates with thrombin generation, the time to reach a predetermined level of clot stiffness (K) and the clotting angle (Angle), which correlates with fibrin polymerization, the maximal amplitude or stiffness (MA), representing clot strength, and the percent of clot breakdown due to fibrinolysis in the first 30 minutes after maximal amplitude (LY30%). The plasma coagulation tests used were PT, aPTT, functional fibrinogen by the modified method of Clauss (STArt 4™ Diagnostica Stago), and D-Dimer measured using ELISA. The lower linear range of detection for fibrinogen for this assay was truncated at 100mg/dl to maintain reliable linear calibration. Fibrinogen clotting times that were prolonged outside of this linear range were assigned the 100mg/dl value.
Platelet contractile force (PCF) was measured using the Hemostasis Analysis System (HAS, Hemodyne Inc. Richmond, VA). The HAS reports viscoelastic properties of static whole blood and is designed to specifically measure the strength of platelet forces during clot contraction (13). Citrated blood was activated by recalcification according to manufacturer directions for HAS measurements.
Definitions and Statistical Methods
Patients were divided into either coagulopathic or non-coagulopathic groups by PT≥ 18 sec. This PT-based definition of coagulopathy was chosen to represent the extent of extrinsic pathway dysfunction and its use in previously-reported trauma cohorts. Fibrinogen was used to represent the degree of consumptive coagulopathy present because it is the first clotting factor to reach critically low levels during bleeding and resuscitation (14). PCF was used to quantify platelet activity since there was little variability in platelet count in this cohort and platelet-specific clotting activity is a strong predictor of outcomes in trauma patients (15). D-Dimer is the plasmin cleavage product of fibrin and has been shown to become elevated in coagulopathic trauma patients and was, therefore, used to represent fibrinolytic activity (16).
The effect of coagulopathy on patient demographics, vital signs, hematology, and TEG parameters was then examined. Base excess as reported in the medical record was transformed to base deficit to simplify interpretation. The total amount of crystalloid (normal saline plus Ringers Lactate) given at the time of study blood sample in the ED and again 8 hours after admission was also analyzed. In addition, the volume of blood products transfused at 8 hours of hospitalization from ED arrival was compared between groups.
Statistical Analysis
Mean and SD were used to describe the data. T tests were used to compare results between coagulopathic and non-coagulopathic groups. As a test of heterogeneity, analysis of means (ANOM) was used to evaluate each TEG parameter individually to identify significant deviations of the mean for each group from the overall cohort mean at alpha=0.05. The highest and lowest quartiles for the TEG parameter MA were then identified and discriminant analysis with forward stepwise variable selection using PT, fibrinogen concentration, PCF, and D-Dimer were used to select which among these variables best discriminated high from low MA. Forward stepwise variable selection with a 0.25 probability to enter was then used to create regression models to determine which coagulation components were most associated with TEG MA. PT, fibrinogen, PCF, D-Dimer, and their interaction terms were made available for forward stepwise variable selection. All statistical analysis was performed using JMP 9.0.0® (SAS, Cary NC, USA)
RESULTS
Of N=173 trauma subjects considered for enrollment, 95 met inclusion criteria and were included in the final analysis. (Figure 1.) Of the included subjects, 91% met criteria for full highest-level trauma team activation. The mean [95%CI] ISS in this cohort was 20 [17, 22], which indicates that the overall injury severity of the cohort could be classified as being “severe” according to current definitions (Severe ISS = 15-25). Blunt injury mechanism accounted for 72% of injuries, 24% were penetrating, and 4% were from combined blunt and penetrating mechanism. Specific mechanisms of injury included 57% from motor vehicle collisions, 22% from gunshots, 15% were pedestrians struck by motor vehicles, 5% were falls, and 1% from stabbing. There was one death in hospital within 72 hours, and 24% of subjects were identified as having traumatic brain injury according to ICD-9 code in the medical record.
Demographics, vital signs, metabolic, and treatment data stratified by coagulopathy are provided in Table 1. Patients with coagulopathy had decreased blood pressure, greater injury severity, and lower GCS scores. They also had more severe metabolic shock as demonstrated by lactate and base deficit. Coagulopathic patients also had received more intravenous crystalloid prior to study blood draw and received more total intravenous fluids, and were transfused a greater volume of blood products by 8 hours of hospitalization. Platelet counts were not different between groups.
Table 1.
Demographics, vital Signs, laboratory, and transfusion.
| Demographics | Overall | PT < 18 seconds | PT ≥ 18 seconds | ||||
|---|---|---|---|---|---|---|---|
| Vitals/Demographics | Mean | SD | Mean | SD | Mean | SD | *T test p value |
| Age (years) | 38.4 | 16.7 | 39.1 | 17.1 | 34.8 | 14.3 | 0.36 |
| Systolic BP (mmHg) | 128.4 | 27.4 | 131.6 | 26.7 | 111.2 | 25.5 | 0.007 |
| Diastolic BP (mmHg) | 79.5 | 18.1 | 81.3 | 17.4 | 69.9 | 19.1 | 0.025 |
| Resp Rate (bpm) | 19.4 | 7.7 | 19.1 | 7.9 | 21.3 | 6.2 | 0.32 |
| Temp C° | 36.4 | 0.8 | 36.5 | 0.8 | 36.1 | 0.6 | 0.23 |
| Oxygen Saturation (%) | 97.4 | 4.1 | 97.5 | 4.2 | 97.1 | 3.5 | 0.72 |
| Pulse Rate (bpm) | 97.0 | 24.4 | 94.0 | 23.2 | 116.7 | 23.6 | <0.001 |
| Injury Severity Score (ISS) | 20 | 13 | 17 | 11 | 32 | 13 | <0.001 |
| Glasgow Coma Score (GCS) | 11 | 5 | 12 | 5 | 9 | 6 | 0.027 |
| Shock Parameters | |||||||
| Lactate (mmol/L) | 4.2 | 2.9 | 3.9 | 2.5 | 5.8 | 4.2 | 0.023 |
| Base Deficit (mEq/L) | 2.7 | 4.7 | 1.9 | 4.0 | 7.2 | 5.8 | <0.001 |
| Hematology | |||||||
| Hemoglobin (g/dl) | 12.3 | 2.0 | 12.7 | 1.8 | 10.5 | 2.2 | <0.001 |
| Hematocrit (%) | 37.3 | 6.4 | 38.4 | 5.7 | 31.3 | 7.0 | <0.001 |
| Platelet Count (×10^9/L) | 237.3 | 74.0 | 241.9 | 74.7 | 213.0 | 66.7 | 0.17 |
| Crystalloid Fluid Resuscitation (ml) | |||||||
| Total Crystalloid Vol. at Sample Time | 1386.2 | 1166.6 | 1275.9 | 1122.7 | 2030.8 | 1254.6 | 0.03 |
| Total Crystalloid Vol. at 8 hours | 2051.0 | 2436.3 | 1699.5 | 1763.4 | 3781.2 | 4167.0 | 0.004 |
| Blood Products (ml) | |||||||
| Total Blood Products at 8 hours | 551.3 | 1617.4 | 255.1 | 677.0 | 2131.0 | 3435.6 | <0.001 |
| Packed Red Blood Cells | 459.1 | 1067.8 | 245.7 | 528.2 | 1526.2 | 2085.2 | <0.001 |
| Fresh Frozen Plasma | 161.3 | 525.1 | 64.6 | 236.3 | 645.0 | 1080.6 | <0.001 |
| Pooled Platelets | 20.5 | 76.2 | 0.0 | 0.0 | 123.1 | 153.6 | <0.001 |
| Apheresis Platelets | 10.3 | 90.6 | 0.0 | 0.0 | 61.5 | 221.9 | 0.024 |
| Cryoprecipitate | 18.8 | 93.8 | 2.3 | 18.6 | 101.5 | 213.9 | <0.001 |
T test comparing PT<18 sec group vs. PT≥18 sec group.
TEG and coagulation measurements are given in Table 2. PT was significantly prolonged in the coagulopathy group indicating good stratification by our definition of coagulopathy (PT>18 sec). Fibrinogen was significantly decreased with coagulopathy and PCF was moderately decreased. Of TEG parameters, only MA was significantly different with coagulopathy compared to non-coagulopathy.
Table 2.
Coagulation parameters and thrombelastography.
| Overall | PT < 18 seconds | PT ≥ 18 seconds | |||||
|---|---|---|---|---|---|---|---|
| Measurement | Mean | SD | Mean | SD | Mean | SD | *T test p value |
| Prothrombin Time (sec) | 15.3 | 2.6 | 14.4 | 1.5 | 20.0 | 1.6 | <.001 |
| Fibrinogen (mg/dl) | 252.8 | 93.9 | 270.4 | 90.1 | 159.1 | 46.9 | <.001 |
| Platelet Contractile Force (Kdynes) | 7.9 | 2.3 | 8.1 | 2.2 | 6.9 | 2.6 | 0.054 |
| D-dimer (ng/ml) | 1596.0 | 1521.8 | 1532.4 | 1519.9 | 1989.0 | 1545.6 | 0.36 |
| Thrombelastography | |||||||
| R (min) | 4.4 | 1.1 | 4.3 | 1.1 | 4.5 | 1.1 | 0.49 |
| K (min) | 1.4 | 0.4 | 1.4 | 0.3 | 1.5 | 0.4 | 0.36 |
| ANG (°) | 68.2 | 6.3 | 68.5 | 5.8 | 66.4 | 8.3 | 0.22 |
| MA (mm) | 61.9 | 5.2 | 62.7 | 4.9 | 57.5 | 4.7 | <.001 |
| LY30 (%) | 1.0 | 1.6 | 1.0 | 1.5 | 1.4 | 2.1 | 0.34 |
T test comparing PT<18 sec group vs. PT≥18 sec group.
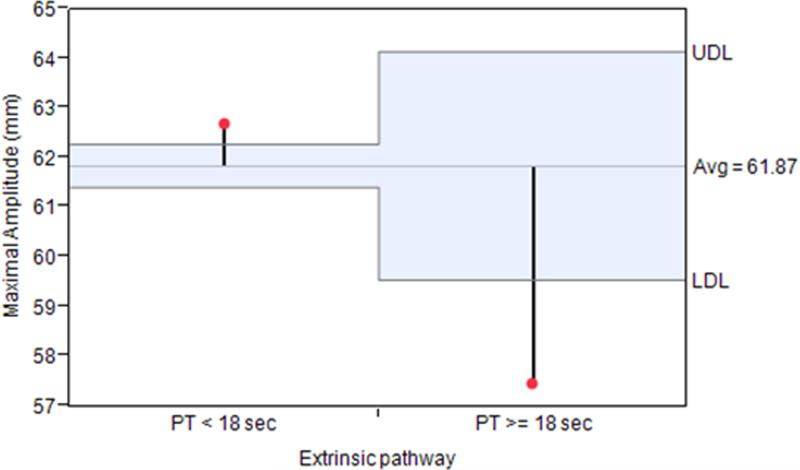
ANOM for each TEG parameter revealed that only MA deviated significantly from the overall cohort mean when stratified into coagulopathic and non-coagulopathic groups. (Figure 2.)
Figure 2.
Analysis of means for TEG maximal amplitude after grouping into coagulopathic (PT≥ 18 sec) and non-coagulopathic groups. Maximal amplitude varied significantly from the overall cohort mean by crossing the predetermined upper decision limit (UDL) in the non-coagulopathic group and by crossing the lower decision limit (LDL) in the coagulopathic group at alpha=0.05.
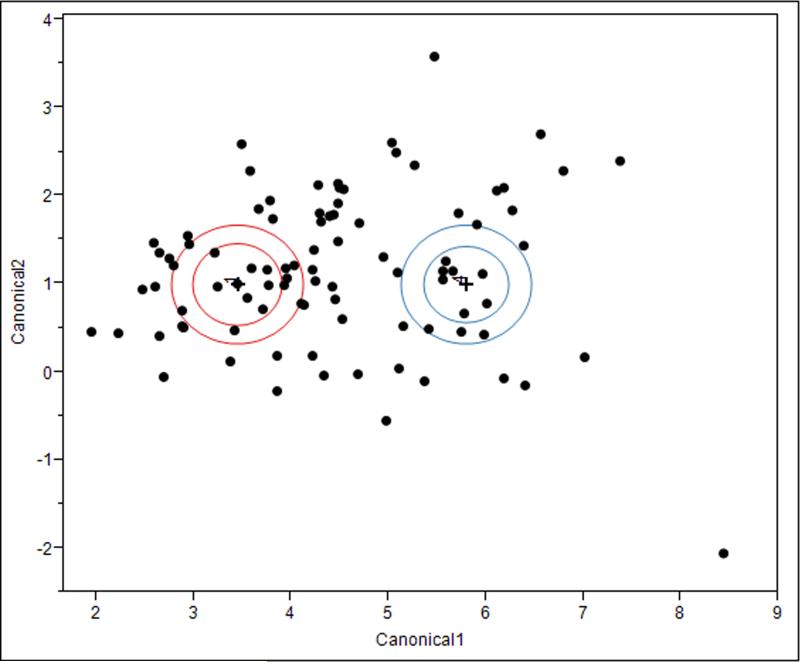
Stepwise selection of coagulation parameters to discriminate high vs. low MA resulted in only fibrinogen (p<0.001) and PCF (p=0.007) meeting criteria for selection into the discriminant model. Subsequent discriminant analysis of the ability of fibrinogen and PCF to discriminate low vs. high MA resulted in clear separation of the two groups with a low 6.7% misclassification rate. (Figure 3.)
Figure 3.
Linear discriminant analysis canonical plot of TEG maximal amplitude quartiles 1 and 4 discriminated by fibrinogen concentration and platelet contractile force measurements. The multivariate mean for each quartile is a labeled inner circle corresponding to a 95% confidence limit for the mean. Groups that are significantly different tend to have non-intersecting circles. Larger contours show areas that contain roughly 50% of the points for that group. Fibrinogen and PCF can clearly discriminate the lowest from highest MA quartiles in this cohort with only 6.7% misclassified.
Fibrinogen concentration, PCF, and their interaction term were selected by forward stepwise variable selection for inclusion in the regression model for TEG MA. The overall model performed moderately well (whole model R2=0.64, p<0.001) and MA was primarily influenced by independent effects of fibrinogen (p<0.001) and PCF (p<0.001) without a significant interaction being present (p=0.9).
DISCUSSION
Using extrinsic pathway dysfunction as a definition of coagulopathy, we found that TEG MA was the most heterogeneous of all TEG parameters. The extent of anatomical injury, blood loss, degree of shock, and other unknown genetic and environmental factors likely define the individual coagulation response to trauma. This study indicates that platelets and fibrinogen should be the primary targets of hemostatic therapy for severely-injured Emergency Department trauma patients.
Our results support the hypothesis that heterogeneity in clot formation early after trauma is primarily reflective of changes in fibrinogen, platelet activity, and less-so fibrinolytic activity. Previous literature suggests that patients with severe injury and shock tend to rapidly become hypocoagulable from a mixture of extrinsic pathway dysfunction by activation of the protein C anticoagulant system (9), consumption of fibrinogen and other clotting factors (17), impaired thrombin generation (18), platelet dysfunction (15), and fibrinolysis (12). However, the relative contribution of these processes to the overall clot formation profile is unclear. Our results provide some clarity regarding the relative importance of these individual components to overall clot formation. Our data agrees with previous publications demonstrating that measured of clot amplitude are the first VHA parameters to become abnormal after trauma (10). There was no difference in TEG onset (R-time) with coagulopathy even when stratifying by extrinsic pathway dysfunction. The lack of a prolongation of TEG-R time with coagulopathy suggests that either anticoagulation was not present in our cohort, PT and aPTT are more reflective of fibrinogen concentration, or that enzymatic anticoagulation in plasma was compensated by the cells present in whole blood.
Our data suggests that perhaps early changes in whole blood clot formation during PCoT may be more simply examined in terms of the balance between fibrin and platelets. Platelets form the initial wound plug by their adhesion and aggregation and are important for thrombin generation. Once sufficient thrombin is generated at the platelet surface, a fibrin scaffold is created that adds stiffness and structure to the forming hemostatic clot. Both formation of an adequate fibrin fiber network and forceful contraction of this network by platelets are required to achieve optimal clot strength. Similar to our results, Kornblith et al, found a consistent decrease in the fibrin-specific contribution to clot strength that was associated with coagulopathy and poor outcomes after trauma (19). They also noted that platelets were the major contributors to maximal clot strength, again suggesting a critical role for platelet-fibrin interactions during clot formation after trauma. Fibrinogen supplementation can compensate for decreased platelet activity and/or count while the converse has not yet been confirmed (20-22). Therefore, focusing on the behavior and interaction of platelets and fibrinogen/fibrin after trauma may provide valuable insight into the mechanism, and provide therapeutic approaches to bleeding management for PCoT.
An important result from this study was that D-Dimer was increased in the entire trauma cohort and it did not differ in the presence of coagulopathy. In addition, clot lysis (TEG-LY30%) was also not different with coagulopathy in our cohort. Kutcher et al., identified a distinct coagulopathy component related to hyperfibrinolysis in trauma patients using principle component analysis. This component was distinct from other hypocoagulable states that were more characteristic of generalized clotting factor consumption and anticoagulation (23). Our results suggest that clot lysis after trauma is likely strongly modulated by clot structure as a result of the interaction between fibrin and platelets.
Limitations
This study is limited in several ways. Firstly, our definition of coagulopathy using extrinsic pathway dysfunction may be overly-simplistic, given the known multifactorial nature of coagulopathy. For future work, techniques such as bioinformatic and hierarchical clustering analysis similar to the approach used to identify complex metabolic states in critically-injured patients may be useful to better discriminate between individual coagulopathic states present after trauma (24). Further investigation into these relationships using non-arbitrary clustering analyses could better identify distinct subgroups of coagulation responses and better understand their clinical implications. Next, subjects received a significant amount of intravenous crystalloid resuscitation fluids prior to sample blood draw. This dilution limits our ability to directly compare this cohort with others where blood samples were obtained prior to fluid resuscitation. The amount of crystalloid fluid received is likely a consequence of prehospital and Emergency Department providers initiating fluid resuscitation according to local protocols. Those patients with a hypocoagulable activation state received significantly more crystalloid intravenously prior to the study blood draw, so our cohort may represent PCoT with secondary dilutional effects. However, we would have expected prolonged clot onset (TEG-R time) in the presence of significant hemodilution, which was not apparent. Next, TBI can make unique contributions to coagulopathy and we did not exclude subjects with TBI from the analysis. Therefore, we cannot separate independent effects of TBI in this cohort. However, TBI is often present after major blunt trauma, and a pragmatic understanding of coagulopathy in the setting of polytrauma with TBI and blood loss is essential for care providers. We also excluded those subjects who were on anticoagulant or antiplatelet medications or had received urgent blood products prior to obtaining the study blood sample. These subjects are also the most likely to require massive transfusion and urgent surgery, and are more likely to die. These exclusions allowed for a more direct examination of coagulopathy after trauma, but also likely masked the emergence of strong anticoagulant profiles or hyperfibrinolysis that are more likely to occur in the most severely injured and shocked trauma patients.
CONCLUSION
TEG MA was the most heterogeneous clot formation parameter present in severely-injured Emergency Department trauma patients and was linked to variation in platelet function, fibrinogen, and less-so fibrinolytic activation. Emergency Department treatment of PCoT should prioritize treatment of fibrinogen and platelets to restore clot strength.
Acknowledgements
This study was supported by the U.S. Defense Health Program, U.S. Army Medical Research and Materiel Command grant W81XWH-11-2-0089. NJW is supported in part, by the National Center for Advancing Translational Sciences (NCATS) (grant KL2 TR000421), a component of the National Institutes of Health (NIH). The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Army and Defense, or the US Government, and do not necessarily represent the official view of NCATS or NIH.
Footnotes
The authors report no conflicts of interest related to this manuscript.
REFERENCES
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JF, Ritenour AE, McLaughlin DF. Injury severity and causes of death from operation Iraqi freedom and operation enduring freedom: 2003-2004 versus 2006. J Trauma Acute Care Surg. 2008;64(2):S21–S26. doi: 10.1097/TA.0b013e318160b9fb. [DOI] [PubMed] [Google Scholar]
- 3.Evans JA, van Wessem KJP, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of Traumatic Deaths: Comprehensive Population-Based Assessment. World Journal of Surgery. 2010;34(1):158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, DePasquale M, Doughty H, Glassberg E, Hervig T, Hooper TJ, Kozar R, Maegele M, Moore EE, Murdock A, Ness PM, Pati S, Rasmussen T, Sailliol A, Schreiber MA, Sunde GA, van de Watering LM, Ward KR, Weiskopf RB, White NJ, Strandenes G, Spinella PC. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. 2014;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess JR, Brohi K, Dutton RP. The Coagulopathy of Trauma: A Review of Mechanisms. J Trauma Acute Care Surg. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 6.Niles SE, McLaughlin DF, Perkins JG. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma Acute Care Surg. 2008;64(6):1459–1463. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 7.Maegele M, Lefering R, Yucel N. Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion Crit Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 9.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet J. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of Surgery. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davenport R, Manson J, De'Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, Stanworth S, Brohi K. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–8. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nystrup KB, Windelov NA, Thomsen AB, Johansson PI. Reduced clot strength upon admission, evaluated by thrombelastography (TEG), in trauma patients is independently associated with increased 30-day mortality. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2011;19:52. doi: 10.1186/1757-7241-19-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thromboelastometry. J Trauma Acute Care Surg. 2007;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 13.Carr ME. Development of platelet contractile force as a research and clinical measure of platelet function. Cell biochemistry and biophysics. 2003;38(1):55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 14.Hiippala ST, Myllyki GJ, Vahtera EM. Hemostatic Factors and Replacement of Major Blood Loss with Plasma-Poor Red Cell Concentrates. Anesth Analg. 1995;81:360–5. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De'Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 17.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 18.Yanagida Y1, Gando S, Sawamura A, Hayakawa M, Uegaki S, Kubota N, Homma T, Ono Y, Honma Y, Wada T, Jesmin S. Normal prothrombinase activity, increased systemic thrombin activity, and lower antithrombin levels in patients with disseminated intravascular coagulation at an early phase of trauma: comparison with acute coagulopathy of trauma-shock. Surgery. 2013;154(1):48–57. doi: 10.1016/j.surg.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255–6. doi: 10.1097/TA.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenkman B, Einav Y, Livnat T, Budnik I, Martinowitz U. In vitro evaluation of clot quality and stability in a model of severe thrombocytopenia: effect of fibrinogen, factor XIII and thrombin-activatable fibrinolysis inhibitor. Blood Transfus. 2014;12(1):78–84. doi: 10.2450/2013.0068-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenkman B, Livnat T, Misgav M, Budnik I, Einav Y, Martinowitz U. The in vivo effect of fibrinogen and factor XIII on clot formation and fibrinolysis in Glanzmann's thrombasthenia. Platelets. 2012;23(8):604–10. doi: 10.3109/09537104.2011.642031. [DOI] [PubMed] [Google Scholar]
- 22.Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, Klingler A, Klima G, Martinowitz U, Fries D. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5(5):1019–25. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 23.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–9. doi: 10.1097/TA.0b013e31828b7fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MJ, Grossman AD, Morabito D, Knudson MM, Butte AJ, Manley GT. Identification of complex metabolic states in critically injured patients using bioinformatic cluster analysis. Crit Care. 2010;14(1):R10. doi: 10.1186/cc8864. [DOI] [PMC free article] [PubMed] [Google Scholar]