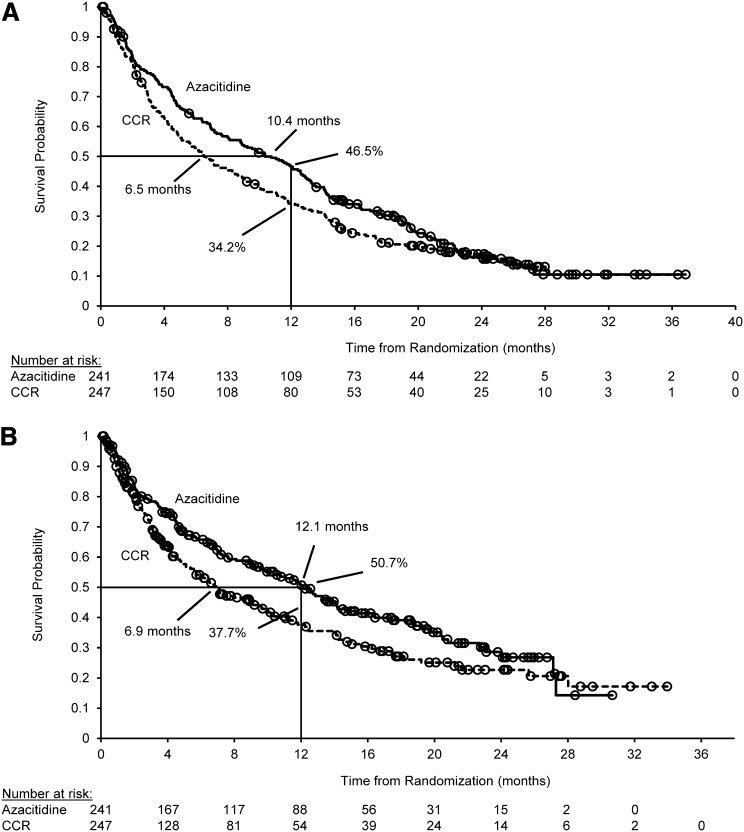
Figure 1.
Primary and preplanned sensitivity analyses for OS of AML therapy. (A) Primary analysis: OS for the intention-to-treat population. Median OS was 10.4 months (95% CI, 8.0-12.7 months) for the azacitidine arm and 6.5 months (95% CI, 5.0-8.6 months) for the CCR arm. In the analysis stratified by ECOG PS and cytogenetic risk, the HR was 0.85 (95% CI, 0.69-1.03; log-rank P = .1009). One-year survival was 46.5% for the azacitidine arm and 34.2% for the CCR arm (difference, 12.3%; 95% CI, 3.5%-21.0%). Median follow-up for OS was 24.4 months. There were 193 deaths in the azacitidine arm (80.1%) and 201 deaths in the CCR arm (81.4%). (B) Preplanned sensitivity analysis: OS censored for subsequent AML therapy (67 azacitidine patients and 75 CCR patients were censored at the time they received subsequent AML therapy). Median OS was 12.1 months (95% CI, 9.2-14.2 months) for the azacitidine arm and 6.9 months (95% CI, 5.1-9.6) for the CCR arm. In the analysis stratified by ECOG PS and cytogenetic risk, the HR was 0.76 (95% CI, 0.60-0.96; log-rank P = .0190). CIs for the difference in 1-year survival probabilities were derived by using Greenwood’s variance estimate. (○) Censored patient.