Abstract
Infection with the nontyphoidal Salmonella is a common cause of food-borne disease that leads to acute gastroenteritis/diarrhea. Severe/prolonged cases of Salmonella infection could also impact host nutritional status, but little is known about its effect on intestinal absorption of vitamins, including biotin. We examined the effect of Salmonella enterica serovar Typhimurium (S. typhimurium) infection on intestinal biotin uptake using in vivo (streptomycin-pretreated mice) and in vitro [mouse (YAMC) and human (NCM460) colonic epithelial cells, and human intestinal epithelial Caco-2 cells] models. The results showed that infecting mice with wild-type S. typhimurium, but not with its nonpathogenic isogenic invA spiB mutant, leads to a significant inhibition in jejunal/colonic biotin uptake and in level of expression of the biotin transporter, sodium-dependent multivitamin transporter. In contrast, infecting YAMC, NCM460, and Caco-2 cells with S. typhimurium did not affect biotin uptake. These findings suggest that the effect of S. typhimurium infection is indirect and is likely mediated by proinflammatory cytokines, the levels of which were markedly induced in the intestine of S. typhimurium-infected mice. Consistent with this hypothesis, exposure of NCM460 cells to the proinflammatory cytokines TNF-α and IFN-γ led to a significant inhibition of biotin uptake, sodium-dependent multivitamin transporter expression, and activity of the SLC5A6 promoter. The latter effects appear to be mediated, at least in part, via the NF-κB signaling pathway. These results demonstrate that S. typhimurium infection inhibits intestinal biotin uptake, and that the inhibition is mediated via the action of proinflammatory cytokines.
Keywords: Salmonella infection, biotin, intestine, colon, transport
biotin is an indispensable micronutrient for normal human health, because it is essential for normal cellular metabolism and function. The vitamin acts as a cofactor for five carboxylases that are critical for the metabolism of fatty acids, glucose, and amino acids (reviewed in Refs. 30, 43, 49). Recent studies have also reported a role for biotin in the regulation of gene expression, where expression of more than 2,000 human genes appears to be affected by biotin status (40, 42, 43, 53, 54). Additionally, biotin appears to play a role in the regulation of immune function (1, 4, 14, 17, 24–26, 33, 41, 43) and in the maintenance of normal intestinal homeostasis (16). Deficiency and suboptimal levels of biotin lead to a variety of clinical abnormalities, including dermal abnormalities and neurological disorders (6, 43, 49). Furthermore, at least in animals, biotin deficiency during pregnancy can lead to embryonic growth retardation, congenital malformation, and death (31).
Since mammals, including humans, cannot synthesize biotin, they must obtain the vitamin from exogenous sources via intestinal absorption. The intestinal tract is exposed to two sources of biotin: dietary biotin, and biotin generated by the gut microbiota. Absorption of biotin in both the small and large intestines occurs via a carrier-mediated process that involves the sodium-dependent multivitamin transporter (SMVT). A variety of conditions appear to affect the intestinal absorption process of biotin (43a), but nothing is known about the effect of enteric pathogens, like Salmonella, on the process. Salmonella enterica is a gram-negative, facultative intracellular bacterium that is capable of infecting a number of different hosts, including humans and mice, and causes significant morbidity and mortality worldwide (8, 19, 20, 34, 37, 56). Salmonellosis is one of the most common food- and water-borne diseases, causing ∼20% of all such infections in humans. Of the 2,500 S. enterica serovars that cause disease in humans, S. typhimurium and S. enteritidis are the most common isolates. S. typhimurium infection in humans leads to acute gastroenteritis that is associated with inflammatory diarrhea (8, 20, 34, 56). Severe and prolonged cases of S. typhimurium infection, as well as dual infection with other pathogens, may also compromise the nutritional status of the infected hosts (12).
During intestinal infection with S. typhimurium, the bacterium exploits M cells and enterocytes/colonocytes (and possibly dendritic cells) to traverse to the subepithelial compartment (29, 46), where it interacts with, and activates, immune cells (macrophages, neutrophils, dendritic cells), leading to production of proinflammatory cytokines (36). Of the secreted cytokines, TNF-α and IFN-γ play important roles in initiating the inflammatory response (8, 18, 20, 34, 46). These cytokines, however, also have the potential of affecting intestinal absorptive and secretory functions (20, 56). In this study, our goal was to examine the effect of S. typhimurium infection on intestinal biotin uptake and to shed light onto the mechanism(s) involved. By using a combination of in vitro and in vivo models, we found that S. typhimurium infection significantly inhibits intestinal biotin uptake. Moreover, we present evidence that the inhibition is indirect and likely dependent on the upregulation of proinflammatory cytokines that act via the NF-κB-mediated signaling pathway, leading to suppression of transcription of SLC5A6, the gene encoding for the SMVT transporter.
MATERIALS AND METHODS
Materials
3H-biotin (specific activity 60 Ci/mmol; radiochemical purity > 97%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). All other cell culture reagents and other chemicals used in this study were of molecular biology grade and were obtained from commercial sources.
Methods
Cell culture.
Confluent monolayers of the human-derived colonic epithelial NCM460 cells, conditionally immortalized murine colonic epithelial cells [young adult mouse colonic (YAMC)] cells, and the human-derived intestinal epithelial Caco-2 cells were used in these investigations (American Type Culture Collection, Manassas, VA). NCM460 cells were maintained in F-12 medium (Ham), whereas Caco-2 and YAMC cells were maintained in MEM and DMEM (Gibco) medium, respectively, supplemented with fetal bovine serum and streptomycin (10 mg/l), under standard condition. Confluent cell monolayers (at 5–6 days postconfluence) were used to examine the effect of cytokines on biotin uptake.
Bacterial Culture and Infection
Salmonella enterica serovar Typhimurium strain IR715 [a nalidixic-acid resistant derivative of strain ATCC 14028 (21)] and an isogenic noninvasive, nonpathogenic mutant [SPN487; invA spiB (37)] were used. For the in vivo studies, C57BL/6 mice were treated with streptomycin (20 mg/mouse) to induce cecal and colonic inflammation (5) and then challenged with S. typhimurium wild-type or with the invA spiB mutant (100 μl of 109 bacteria/ml). Mice were killed 72 h later, and their intestinal tissues were collected and analyzed.
For the in vitro studies, overnight bacterial cultures were grown in Luria-Bertani broth, washed with PBS, and diluted to a multiplicity of infection = 10 in the respective cell culture medium without fetal bovine serum and antibiotics. Following a 90-min infection, the intestinal epithelial monolayers were treated with gentamycin (50 μg/ml) to remove adherent bacteria (15), allowed to recover for 6 h, and used for biotin uptake investigations.
Biotin Uptake
For mouse studies, we used the in vivo intestinal (jejunal) closed-loop technique (16), as well as the isolated colonic sheet approach (48). In the former studies, we introduced 83 nM 3H-biotin into the loop, followed 5 min later (after animal was killed) by removal of loop tissue, processing, and counting of the 3H content (using liquid scintillation counter). In the colonic sheet investigations, equal pieces (1 cm) of colonic sheets were incubated (5 min) in vitro in Krebs-Ringer (KR; pH 7.4) buffer in the presence of 64 nM 3H-biotin, washed, and then processed for 3H content.
For the studies with cultured cells, confluent monolayers of NCM460, YAMC, and Caco-2 cells treated with heat-stable toxins were used, as described previously (15). In brief, cells were incubated (5 min; initial rate) in KR buffer (pH 7.4) at 37°C in the presence of 5 nM 3H-biotin. The cells were then washed twice with ice-cold KR buffer, lysed with 1 N NaOH (followed by neutralization with 10 N HCl), and counted for radioactivity in a liquid scintillation counter.
Real-Time PCR Analysis
Total RNA was isolated from infected cells or mice tissues in TRIzol (Life Technologies), as described in the manufacturer's protocol, and then treated with DNase. The RNA pool was reverse transcribed to cDNA using i-script kit (Bio-Rad) and then used for quantitative real-time PCR using specific primers [mSlc5a6 F: 5′-GGATCTGTGGGACTGTGA-3′; R: 5′-CACATCTGTCCAGATGACA-3′; mβ-actin, F: 5′-ATCCTCTTCCTCCCTGGA-3′, R: 5′-TTCATGGATGCCACAGGA-3′; hSLC5A6 F: 5′-TGTCTACCTTCTCCATCATGGA-3′, R: 5′-TAGAGCCCAATGGCAAGAGA-3′; hβ-actin F: 5′-CATCCTGCGTCTGGACCT-3′, R: 5′-TAATGTCACGCACGATTTCC-3′; heterologous nuclear RNA (hnRNA)-mSlc5a6 F: 5′-ATCTCACCAGTGCCTATGA-3′, R: 5′-CACACTGAGTTTTCTGTCC-3′; hnRNA-β actin F: 5′-AGATGACCCAGGTCAGTATC-3′, R: 5′-GAGCAGAAACTGCAAAGAT-3′; mIfnγ F: 5′-TCAAGTGGCATAGATGTGGAAGAA-3′, R: 5′-TGGCTCTGCAGGATTTTCATG-3′; mTnf-α F: 5′-AGCCCCCAGTCTGTATCCTT-3′, R: 5′-CTCCCTTTGCAGAACTCAGG-3′] in a CFX96 real-time i-cycler (Bio-Rad). The relative expression of Slc5a6 or Tnf-α or Ifnγ was normalized to β-actin as internal control and quantified by 2−ΔΔCt method (28).
Western Blot Analysis
For Western blotting, mouse tissues and cultured cells were homogenized in RIPA buffer (Sigma) in the presence of a complete protease inhibitor cocktail (Roche, Nutley, NJ). The soluble fraction was isolated by centrifugation at 8,000 g for 5 min, and the protein content was measured using the DC protein assay kit (Bio-Rad). An equal amount of protein was loaded onto a 10% mini-gel (Invitrogen) and transferred to polyvinylidene difluoride membrane for Western blot analysis. The membrane was blocked overnight and probed with specific antibodies for 3 h, followed by corresponding secondary antibodies (LI-COR Biosciences, Lincoln, NE) in 1:25,000 dilution for 1 h at room temperature. Relative expression of the gene was quantified by comparing the fluorescent intensities with respect to corresponding β-actin using Odyssey application software (version 3.0) in an Odyssey Infrared imaging system (LI-COR).
Promoter Activity: Transfection and Reporter Gene Assay
Activity of the SLC5A6 promoter fused to luciferase reporter construct was determined as described previously (11). In brief, NCM460 cells were cotransfected with the SLC5A6 promoter construct (3 μg) and the control plasmid of Renilla luciferase-thymidine kinase (100 ng) (Promega, Madison, WI), followed 24 h later by treatment with IFN-γ (30 ng/ml) and TNF-α (20 ng/ml) for an additional 24 h. Luciferase activity was then determined using Dual Luciferase Assay system (Promega).
Isolation of Cytosolic and Nuclear Fractions
Cytosolic and nuclear fractions were isolated using the NE-PER Nuclear and Cytosolic Extraction Kit following the directions provided by the manufacturer (Thermo Scientific). Both fractions were stored at −80°C freezer before use.
Cytokine Determination
Tnf-α and Ifnγ released in mouse serum were determined by ELISA, following the manufacturer protocol (OptEIA Kit, BD Biosciences). Briefly, 100 μl of serum were added to each well, coated with the appropriate cytokine capture antibody. After washing, specific antibodies were added to the corresponding wells. The relative abundance of the cytokines in the serum sample was measured colorimetrically using tetramethylbenzidine and detected in a microplate reader (VERSAmax microplate reader, Molecular Devices).
Statistical Analysis
All uptake data presented in the study are means ± SE of multiple separate determinations. Uptake was expressed as femtomoles per milligram protein per 5 min. Biotin uptake by the carrier-mediated process was determine by subtracting uptake of a physiological concentration of 3H-biotin in the presence of a high pharmacological concentration of unlabeled biotin (1 mM) from uptake in its absence. All other determinations are presented as means ± SE of at least three independent experiments; promoter activity (in arbitrary units) is expressed as fold over pGL3-Basic. Statistical significance was determined from Student's t-test or one-way ANOVA, with statistical significance set at <0.05.
RESULTS
S. typhimurium Infection Inhibits Intestinal Biotin Uptake by Downmodulating the Expression of the Vitamin Transporter SMVT
To determine the effect of S. typhimurium infection on intestinal biotin uptake, we infected C57BL/6 mice with wild-type S. typhimurium by oral gavage. At 72 h postinfection, we determined 3H-biotin uptake in the small intestine and in the colon compared with sex- and age-matched control mice gavaged with PBS. Our results showed a significant inhibition of 3H-biotin uptake, both in the small intestine and in the colon (P < 0.01 for both) (Fig. 1, A and B). In contrast, we did not observe any changes in jejunal and colonic biotin uptake when mice were gavaged with a S. typhimurium ΔinvA ΔspiB mutant, which is avirulent because it does not invade the intestinal mucosa nor replicate within the host (Fig. 1C and data not shown). As our results indicated that S. typhimurium infection decreases biotin uptake, we next investigated whether this effect was dependent on the inhibition of the vitamin transporter SMVT, which our laboratory recently found to be the only biotin uptake system in the intestinal tract (16). To this end, we examined whether S. typhimurium infection would reduce the expression of the Slc5a6 mRNA and the SMVT protein in the jejunum and in the colon. Our results (Fig. 2) show a significant (P < 0.01) decrease in the level of expression of both the Slc5a6 mRNA and the SMVT protein in the jejunum and colon of S. typhimurium-infected mice, compared with uninfected controls.
Fig. 1.
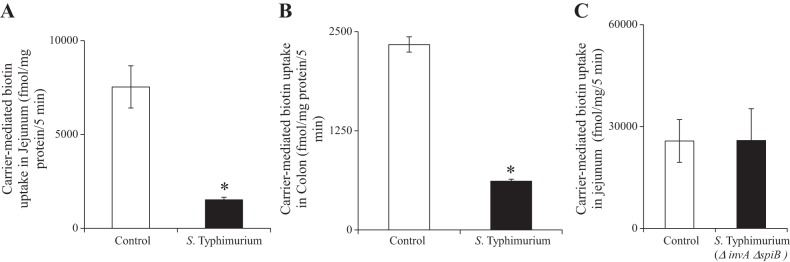
Effect of Salmonella infection on intestinal biotin uptake in vivo. A: carrier-mediated in vivo biotin uptake in jejunal loops in sex-matched littermates. B: carrier-mediated biotin uptake in colonic sheets of infected mice and sex-matched littermate controls. C: carrier-mediated biotin uptake after infection with the nonpathogenic S. typhimurium ΔinvA ΔspiB mutant in vivo. Values are means ± SE of 3–6 independent observations from 3–6 different sets of mice. *P < 0.01.
Fig. 2.
Expression of the Slc5a6 gene and of the sodium-dependent multivitamin transporter (SMVT) protein in jejunum and colon of Salmonella-infected mice. A: quantitative PCR using reverse transcribed total RNA from jejunum (i) and colon (ii) of control and S. typhimurium-infected mice. B: Western blot analysis of the SMVT protein expression in jejunal (i) and colonic (ii) mucosa. Values are means ± SE of at least 3 separate sets of mice. *P < 0.01.
Because changes in the mRNA of a given gene could be mediated by changes in the transcription rate of that gene, we examined the effect of S. typhimurium infection on the expression levels of the endogenous Slc5a6 hnRNA; the level of hnRNA reflects rate of transcription (13, 47). We found a significant (P < 0.01) decrease in the level of Slc5a6 hnRNA in the jejunum of the S. typhimurium-infected mice compared with controls (Fig. 3), suggesting that S. typhimurium infection reduces the transcription rate of the Slc5a6 gene. Similar changes were also found in the colon of S. typhimurium-infected mice (data not shown). Taken together, our results indicate that S. typhimurium infection reduces the transcription rate of the Slc5a6 gene, resulting in reduced production of the SMVT transporter and, consequently, in reduced biotin uptake during infection.
Fig. 3.
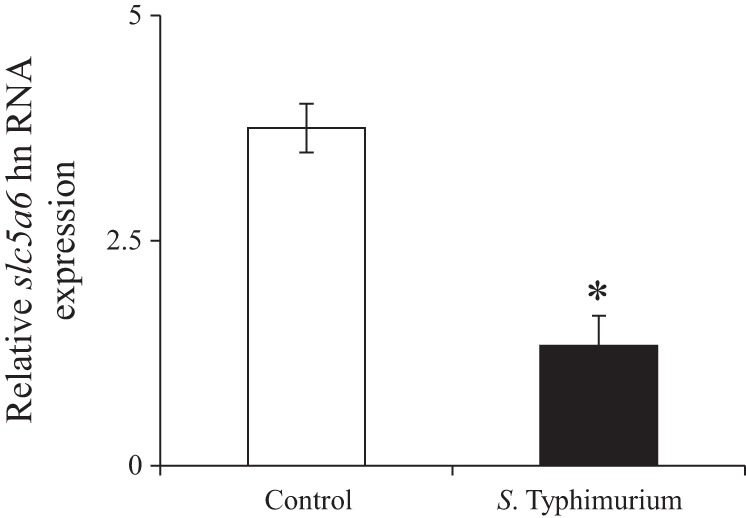
Effect of Salmonella infection on level of expression of the Slc5a6 heterologous nuclear RNA (hnRNA). Quantitative PCR using cDNA reverse transcribed from total RNA from jejunum of S. typhimurium-infected mice and sex-matched littermate controls is shown. Values are means ± SE of at least 3 separate sets of experiments (mice). *P < 0.01.
The Proinflammatory Cytokines Tnf-α and Ifnγ Are Induced During S. typhimurium Infection and Inhibit Biotin Uptake In Vitro
Next, we sought out to determine the mechanisms by which S. typhimurium inhibits the expression of SMVT and biotin uptake. To this end, we set up in vitro culture models, and we infected mouse colonic epithelial YAMC cells, human colonic epithelial NCM460 cells, and confluent Caco2 cells, which resemble enterocytes from the small intestine, with wild-type S. typhimurium (multiplicity of infection = 10) for 90 min. We then measured the initial rate of 3H-biotin (5 nM) uptake. Surprisingly, we detected similar biotin uptake in uninfected cells and in cells infected with S. typhimurium in all of the three cell types used (Fig. 4). These findings suggest that the observed inhibition of jejunal and colonic biotin uptake in mice infected with S. typhimurium is most likely indirect in nature.
Fig. 4.

Effect of Salmonella infection on intestinal biotin uptake in vitro. Mouse colonic YAMC cells, human colonic NCM460 cells, and human Caco-2 enterocytes were serum-starved overnight 3–4 days postconfluence and then infected with wild-type S. typhimurium at a multiplicity of infection of 10. Biotin uptake was subsequently measured as described in materials and methods. A: effect of Salmonella on carrier-mediated biotin uptake on YAMC cells. B: effect of Salmonella on carrier-mediated biotin uptake on NCM460 cells. C: effect of Salmonella on carrier-mediated biotin uptake on Caco-2 cells. Values are means ± SE of at least 3 experiments.
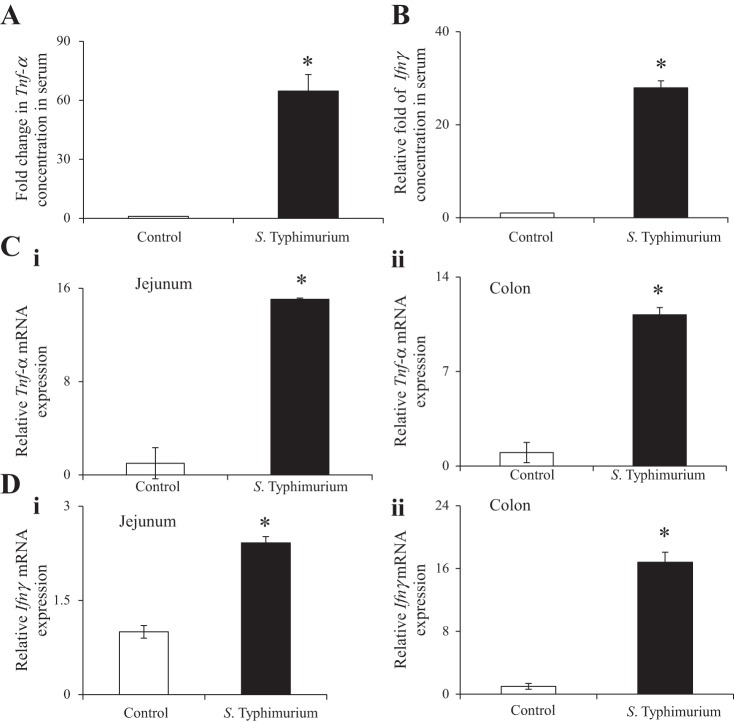
It is known that Salmonella infection in humans leads to an increase in serum levels of proinflammatory cytokines, including that of TNF-α and IFN-γ (46). Exposure of intestinal epithelial cells to elevated levels of proinflammatory cytokines affects the intestinal transport of a variety of substrates (2, 10, 39, 44). It is, therefore, possible that the inhibitory effect of S. typhimurium infection on mouse intestinal biotin uptake in vivo is mediated by an effect of proinflammatory cytokines on the vitamin uptake process. To test this hypothesis, we first determined whether S. typhimurium infection of mice cause an increase of Tnf-α and Ifnγ, two proinflammatory cytokines known to affect intestinal physiology, in both the serum and in the gut mucosa (Fig. 5). We found a significant (P < 0.01; ∼64 and 27-fold for TNF-α and IFN-γ, respectively) induction in serum levels of TNF-α and IFN-γ in S. typhimurium-infected mice, compared with controls (Fig. 5, A and B). Moreover, we also detected a significant (P < 0.01 for both) upregulation of Tnf-α and Ifnγ mRNA in the small intestine and in the colon (Fig. 5, C and D). In contrast, no upregulation of these proinflammatory genes was observed when the mice were infected with the nonpathogenic S. typhimurium ΔinvA ΔspiB mutant (data not shown).
Fig. 5.
Level of Tnf-α and Ifnγ expression in serum and intestinal mucosa of Salmonella-infected mice and sex-matched littermate controls. Fold changes in the serum level of Tnf-α (A) and Ifnγ (B) were measured by ELISA. C: quantitative PCR was performed to determine Tnf-α mRNA expression using total RNA from jejunum (i) and colon (ii). D: quantitative PCR was performed to detect Ifnγ mRNA expression using cDNA prepared from total RNA from jejunum (i) and colon (ii). Values are means ± SE of at least 3–4 separate sets of mice. *P < 0.01.
We next examined the effect of exposure of human colonic epithelial NCM460 cells to TNF-α and IFN-γ on the biotin uptake mechanisms. In these studies, cells were treated with TNF-α and IFN-γ (20 and 30 ng/ml, respectively) for 24 h, followed by the examination of the initial rate of biotin uptake. Consistent with our prediction, we observed a significant (P < 0.01) inhibition of biotin uptake in cells treated with these cytokines compared with untreated controls (Fig. 6A). As we observed in S. typhimurium-infected mice, inhibition of biotin uptake was associated with a significant (P < 0.01) reduction in the levels of expression of the SMVT protein and SLC5A6 mRNA (Fig. 6, B and C). To further examine the mechanisms of biotin-uptake inhibition, we next investigated the effect TNF-α and IFN-γ on the activity of the SLC5A6 promoter; we focused our study on the SLC5A6 promoter-1, since it is the most active in the intestine (11). We found a significant (P < 0.01) decrease in the activity of the SLC5A6 promoter in cells pretreated with the cytokines, compared with untreated controls (Fig. 6D). These findings indicate that the inhibition of intestinal biotin uptake caused by proinflammatory cytokines is, at least in part, mediated at the level of transcription of the SLC5A6 gene.
Fig. 6.
Effect of TNF-α and IFN-γ on biotin uptake, SMVT protein level, and SLC5A6 mRNA expression in NCM460 cells. A: carrier-mediated biotin uptake in colonic NCM460 cells followed by incubation with proinflammatory cytokines (20 ng/ml and 30 ng/ml of TNF-α and IFN-γ, respectively). B: Western blot assay of hSMVT after normalization with β-actin as internal control; inset shows representative gel picture in absence and presence cytokines. C: quantitative real-time PCR to analyze SLC5A6 expression in cytokine-treated NCM460 cells compared with the untreated control. D: effect of cytokines on the SLC5A6-P1 promoter. Relative firefly luciferase activity was normalized by renilla firefly activity and the value expressed as fold over basic. Values are means ± SE of at least 3–4 separate sets of experiments (mice). *P < 0.01.
Role of the NF-κB Pathway in Mediating the Inhibitory Effect of Proinflammatory Cytokines on SLC5A6 Transcription and on Biotin Uptake
One of the important intracellular signaling mechanisms that mediate the effect of proinflammatory cytokines on intestinal epithelial cells is mediated by the NF-κB signaling pathway (32, 55). Also, a computer analysis (Alibaba 2.1; http://www.gene-regulation.com/pub/programs/alibaba2/index.html) showed that the human and mouse SLC5A6 promoters both have a putative site for NF-κB. To determine whether the inhibitory effect of TNF-α and IFN-γ on the activity of the SLC5A6 promoter and on biotin uptake is dependent on NF-κB signaling, we used the NF-κB inhibitor MG132, which blocks activation of NF-κB by preventing the degradation of the NF-κB inhibitor IκB (22, 32). In these investigations, we first confirmed that treatment of NCM460 cells with TNF-α and IFN-γ leads to translocation of the NF-κB subunit p65 into the nucleus and to the degradation of IκB in the cytosol (Fig. 7A, i–ii). We then examined the effect of pretreating NCM460 cells for 24 h with TNF-α and IFN-γ in the presence of the NF-κB inhibitor MG132 (1 μM) on the activity of the SLC5A6 promoter and on biotin uptake (Fig. 7, B and C). Our results showed that NF-κB inhibition significantly (P < 0.01 for both) attenuates the degree of cytokine-mediated inhibition of SLC5A6 promoter activity and of biotin uptake. These findings suggest that activation of the NF-κB pathway mediates the inhibition of intestinal biotin uptake observed during TNF-α and IFN-γ treatment.
Fig. 7.
Role of NF-κB in the inhibitory effect of proinflammatory cytokine on biotin uptake by NCM460 cells: translocation of p65 and degradation of IκB. A: Western blot analysis using nuclear (i) and cytosolic (ii) fractions of cytokine-treated NCM460, showing the relative amounts of NF-κB and IκB, respectively. B: effect of the NF-κB inhibitor, MG132, on the proinflammatory cytokine-induced inhibition on SLC5A6-P1 promoter activity (i) and carrier-mediated biotin uptake (ii). Values are means ± SE of at least 3 separate sets of experiments. *P < 0.05, **P < 0.01.
DISCUSSION
The aim of this study was to examine the effect of Salmonella infection on the uptake of the water-soluble vitamin biotin, and to determine the mechanism(s) involved in the observed effect. Salmonellosis is one of the major public health concerns worldwide, affecting millions of people globally. Prolonged and severe cases of this infection have the potential to negatively impact normal body homeostasis of water-soluble vitamins, including biotin. Thus far, however, no studies have examined the possible effects of Salmonella infection on intestinal absorption of water-soluble vitamins, including biotin. Here we investigated this question, using both in vivo and in vitro models of Salmonella infection: the streptomycin-pretreated mouse model (27, 35), and cultured intestinal and colonic epithelial cell lines.
Our results showed that infection of mice with wild-type S. typhimurium, but not with a noninvasive, nonpathogenic S. typhimurium mutant, leads to a significant inhibition of biotin uptake, both in the small intestine and in the colon. These findings indicate that absorption of both dietary biotin, which is usually absorbed in the small intestine, as well as the biotin that is generated by large intestinal microbiota (3), is inhibited by Salmonella infection. Notably, the inhibition of intestinal biotin uptake was found to be associated with a significant reduction in the expression of the Slc5a6 gene and of its gene product SMVT (SMVT is the only biotin uptake system that operates in the intestinal tract; Ref. 16). The inhibition in SMVT expression in the intestine of S. typhimurium-infected mice appears to be occurring, at least in part, at the level of transcription of the Slc5a6 gene, as suggested by the significant reduction in the expression level of the Slc5a6 hnRNA (level of hnRNA reflects changes in transcription rate of genes).
In contrast to the inhibitory effect in intestinal biotin uptake observed in mice infected with S. typhimurium, no such inhibition was seen during infection of mouse and human intestinal or colonic epithelial cells. Collectively, these in vivo and in vitro findings suggest that the effect of S. typhimurium infection on intestinal biotin uptake is indirect in nature. This idea is consistent with the present understanding of how Salmonella infection affects gut mucosal physiology, i.e., mainly via the proinflammatory response that it elicits (36, 45). To further test this hypothesis in the present investigation, we first determined whether two representative proinflammatory cytokines, TNF-α and IFN-γ, were induced in our S. typhimurium-infected mice. Consistent with the above-described prediction, we found that the levels of both TNF-α and IFN-γ were markedly induced in the intestinal mucosa and in the serum of S. typhimurium-infected mice. We then examined the effect of exposing intestinal epithelial cells to these cytokines and observed a significant inhibition of biotin uptake, as well as inhibition in the level of expression of the SMVT mRNA and activity of the SLC5A6 promoter. The cytokine-mediated inhibition in intestinal biotin uptake and level of expression of the SMVT protein are in contrast to the stimulatory effect they cause in intestinal uptake of peptides and in level of mRNA expression of the involved transporter, pepT1 (9, 52).
Because the NF-κB pathway is a major intracellular hub that mediates inflammatory responses, and because our bioinformatic analysis predicted that the 5′-regulatory region of the SLC5A6 gene contains putative NF-κB sites, we determined whether activation of the NF-κB pathway by TNF-α and IFN-γ would result in the inhibition of biotin uptake by intestinal epithelial cells. As we predicted, our findings showed that inhibition of the NF-κB pathway with the blocker MG132 significantly reverted the inhibitory effect of TNF-α and IFN-γ on both the SLC5A6 promoter activity and on biotin uptake in the human intestinal NCM460 cell line. Together, our results indicate that the proinflammatory cytokines TNF-α and IFN-γ exert their inhibitory effect on intestinal biotin uptake via, at least in part, an NF-κB-dependent down-modulation of the SLC5A6 gene transcription, resulting in reduced expression of the multivitamin transporter SMVT. Moreover, our findings are likely relevant to other conditions that are associated with intestinal inflammation and with an increase of both TNF-α and IFN-γ, like inflammatory bowel diseases; of note, significantly lower biotin levels have been observed in inflammatory bowel disease patients (7, 18, 23, 46).
In conclusion, our findings show that infection with S. typhimurium leads to a significant inhibition of small intestinal and colonic biotin uptake, and that this inhibition is indirect in nature, as it is mediated by TNF-α and IFN-γ, two proinflammatory cytokines highly induced during S. typhimurium infection. Furthermore, the inhibitory effect of these cytokines appears to be, at least in part, exerted at the level of transcription of the SLC5A6 gene via the NF-κB signaling pathway, resulting in lower levels of the SMVT vitamin transporter. Finally, the inhibition of intestinal biotin uptake by proinflammatory cytokines observed in this study may also provide an explanation for the low biotin levels found in patients with chronic intestinal inflammation (14, 52).
GRANTS
This study was supported by grants from the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases (DK58057 and DK-56061). Work in the MR laboratory is supported by Public Health Service Grants AI083663, AI101784, and AI105374, and by Burroughs Wellcome Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.G., S.J., M.R., and H.M.S. conception and design of research; A.G., S.J., and R.K. performed experiments; A.G., S.J., M.R., and H.M.S. analyzed data; A.G., S.J., M.R., and H.M.S. interpreted results of experiments; A.G. prepared figures; A.G., M.R., and H.M.S. drafted manuscript; A.G., S.J., R.K., M.R., and H.M.S. edited and revised manuscript; A.G., S.J., R.K., M.R., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Abad-Lacruz A, Fernandez-Banares F, Cabre E, Gil A, Esteve M, Gonzalez-Huix F, Xiol X, Gassull MA. The effect of total enteral tube feeding on the vitamin status of malnourished patients with inflammatory bowel disease. Int J Vitam Nutr Res 58: 428–435, 1988. [PubMed] [Google Scholar]
- 2.Amin MR, Malakooti J, Sandoval R, Dudeja PK, Ramaswamy K. IFN-gamma and TNF-alpha regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol 291: C887–C896, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J; Meta HIT Consortium, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M'Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature 473: 174–180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baez-Saldana A, Diaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71: 2839–2858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonjour JP. Vitamins and alcoholism. IV. Thiamin. Int J Vitam Nutr Res 50: 321–338, 1980. [PubMed] [Google Scholar]
- 7.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521–533, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Brown M, Eykyn SJ. Non-typhoidal Salmonella bacteraemia without gastroenteritis: a marker of underlying immunosuppression. Review of cases at St Thomas' Hospital 1970–1999. J Infect 41: 256–259, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Charrier L, Sitaraman S, Gewirtz A, Merlin D. Interferon-γ increases hPepTi-mediated uptake of Di-tripeptides including the bacterial tripeptide fMLP in polarized intestinal epithelia. Am J Pathol 163: 1969–1977, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Xu H, Dong J, Li J, Ghishan FK. Tumor necrosis factor-alpha impairs intestinal phosphate absorption in colitis. Am J Physiol Gastrointest Liver Physiol 296: G775–G781, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim Biophys Acta 1574: 187–192, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect 3: 1191–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Elferink CJ, Reiners JJ Jr. Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 15.Ghosal A, Chatterjee NS, Chou T, Said HM. Enterotoxigenic Escherichia coli infection and intestinal thiamin uptake: studies with intestinal epithelial Caco-2 monolayers. Am J Physiol Cell Physiol 305: C1185–C1191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsburg CH, Dambrauskas JT, Ault KA, Falchuk ZM. Impaired natural killer cell activity in patients with inflammatory bowel disease: evidence for a qualitative defect. Gastroenterology 85: 846–851, 1983. [PubMed] [Google Scholar]
- 18.Indaram AV, Visvalingam V, Locke M, Bank S. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am J Gastroenterol 95: 1221–1225, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect 3: 1183–1190, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kalupahana RS, Mastroeni P, Maskell D, Blacklaws BA. Activation of murine dendritic cells and macrophages induced by Salmonella enterica serovar Typhimurium. Immunology 115: 462–472, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsley RA, Humphries AD, Weening EH, De Zoete MR, Winter S, Papaconstantinopoulou A, Dougan G, Baumler AJ. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: identification of intestinal colonization and persistence determinants. Infect Immun 71: 629–640, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8: 739–758, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem 47: 1297–1301, 2001. [PubMed] [Google Scholar]
- 24.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. [DOI] [PubMed] [Google Scholar]
- 25.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-alpha production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1beta production in mice. J Nutr 139: 1031–1036, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11: 227–239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 155: 1319–1321, 1987. [DOI] [PubMed] [Google Scholar]
- 30.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr 133: 2519–2525, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima S, Kato H, Takahashi S, Johno H, Kitamura M. Inhibition of NF-kappaB by MG132 through ER stress-mediated induction of LAP and LIP. FEBS Lett 585: 2249–2254, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Okabe N, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn's disease. Dig Dis Sci 33: 1495–1496, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Pietila TE, Veckman V, Kyllonen P, Lahteenmaki K, Korhonen TK, Julkunen I. Activation, cytokine production, and intracellular survival of bacteria in Salmonella-infected human monocyte-derived macrophages and dendritic cells. J Leukoc Biol 78: 909–920, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5: 476–486, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14: 421–428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology 204: 572–581, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Chavez F, Winter SE, Lopez CA, Xavier MN, Winter MG, Nuccio SP, Russell JM, Laughlin RC, Lawhon SD, Sterzenbach T, Bevins CL, Tsolis RM, Harshey R, Adams LG, Baumler AJ. Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS Pathog 9: e1003267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-gamma downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol 280: C1224–C1232, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Melendez R, Griffin JB, Zempleni J. The expression of genes encoding ribosomal subunits and eukaryotic translation initiation factor 5A depends on biotin and bisnorbiotin in HepG2 cells. J Nutr Biochem 17: 23–30, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Melendez R, Schwab LD, Zempleni J. Jurkat cells respond to biotin deficiency with increased nuclear translocation of NF-kappaB, mediating cell survival. Int J Vitam Nutr Res 74: 209–216, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review). J Nutr Biochem 14: 680–690, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. [DOI] [PubMed] [Google Scholar]
- 43a.Said HM, Trebble TM. Intestinal digestion and absorption of micronutrients. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease. (10th Ed.), edited by M. Feldman, L. S. Friedman, and L. J. Brandt. Philadelphia, PA: Elsevier, 2015, vol. 2, chapt. 103, p 1765–1787. [Google Scholar]
- 44.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos RL, Raffatellu M, Bevins CL, Adams LG, Tukel C, Tsolis RM, Baumler AJ. Life in the inflamed intestine, Salmonella style. Trends Microbiol 17: 498–506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoycheva M, Murdjeva M. Serum levels of interferon-gamma, interleukin-12, tumour necrosis factor-alpha, and interleukin-10, and bacterial clearance in patients with gastroenteric Salmonella infection. Scand J Infect Dis 37: 11–14, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol 299: F28–F34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr 6: 317–343, 1986. [DOI] [PubMed] [Google Scholar]
- 51.Urabe K. [Decreased plasma biotin levels in patients with Crohn's disease]. Nihon Shokakibyo Gakkai Zasshi 83: 697, 1986. [PubMed] [Google Scholar]
- 52.Vavricka SR, Musch MW, Fujiya M, Kles K, Chang L, Eloranta JJ, Kullak-Ublick GA, Drabik K, Merlin D, Chang EB. Tumor necrosis factor-α and interferon-γ increase PepT1 expression and activity in the human colon carcinoma cell line Caco-1/bbe and in mouse intestine. Pflugers Arch 452: b71–b80, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Velazquez-Arellano A. From an inborn error patient to a search for regulatory meaning: a biotin conducted voyage. Mol Genet Metab 87: 194–197, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Wiedmann S, Rodriguez-Melendez R, Ortega-Cuellar D, Zempleni J. Clusters of biotin-responsive genes in human peripheral blood mononuclear cells. J Nutr Biochem 15: 433–439, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem 267: 22506–22511, 1992. [PubMed] [Google Scholar]
- 56.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol 27: 313–320, 2000. [DOI] [PubMed] [Google Scholar]