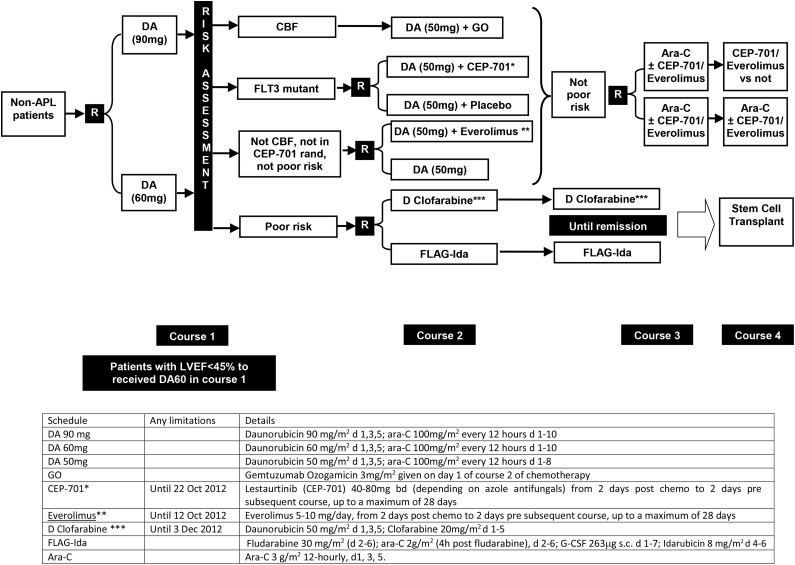
Figure 1.
Trial design of AML17. Patients allocated either CEP-701 or everolimus post–course 1 carried this molecule forward into subsequent courses. *After closure of the CEP-701 randomization, patients were guided by risk score to either poor risk or not poor risk options. **After closure of the everolimus randomization, patients in this group received daunorubicin (DA) 50 mg alone. ***After closure of the D clofarabine arm, patients were recommended to receive FLAG-Ida (which was also the case if renal criteria were not met).