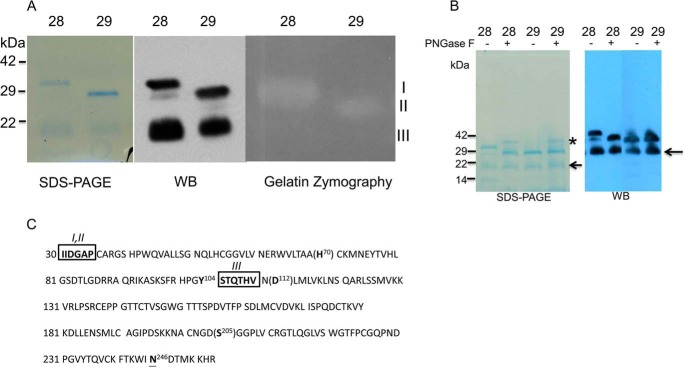
FIGURE 1.
Characterization of purified recombinant active of protein KLK7. Panel A, left, Coomassie-stained reduced SDS-PAGE of purified mat-KLK7 (mat = mature). Two chromatographic fractions are shown, fractions 28 (lanes 1, 3, and 5) and 29 (lanes 2, 4, and 6). Three bands are visible, I (30 kDa), II (28 kDa), and III (20 kDa). All three bands were found to contain KLK7 sequences by mass spectrometry and reacted with anti-KLK7 antibodies on Western blots (WB; middle panel). On gelatin zymography (right panel) only bands I and II had enzymatic activity. Panel B, Coomassie staining of reduced SDS-PAGE (left) and Western blotting analysis (right) of purified mature KLK7 (FPLC fractions 28 and 29) with or without peptide N-glycosidase F (PNGase F) treatment. * refers to peptide N-glycosidase F band, whereas the arrow refers to 20-kDa band of KLK7. Panel C, amino acid sequence of active-form of KLK7. Catalytic amino acids are shown in brackets. The N-terminal sequence of bands I and II was identical (IIDGAP) and in accordance with the sequence of active mature KLK7. The N-terminal sequence of band III was STQTHV, indicating that this form of KLK7 originated via proteolytic (likely autoproteolytic) cleavage after Tyr-104. Boxed amino acids indicate data from N-terminal sequencing and, underlined amino acid N246 is the potential glycosylation site of KLK7.