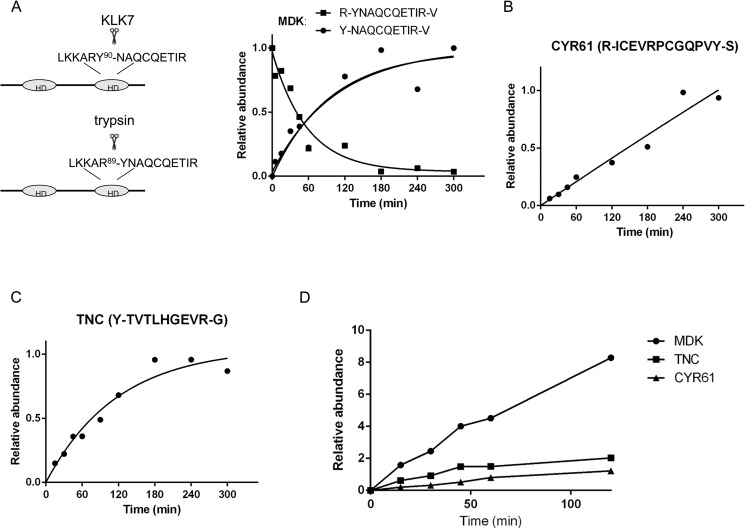
FIGURE 6.
SRM-based time course analysis of KLK7-mediated protein cleavage. Briefly, 0.25 μm concentrations of recombinant MDK (A), CYR61 (B), and TNC (C) were incubated with KLK7 (enzyme:substrate ratio of 1:100 (w/w)) at various time points at 37 °C. The reaction was stopped, and the peptides were monitored using the SRM assay. D, 0.25 μm concentrations of each substrate were mixed and incubated with KLK7 (enzyme:substrate ratio of 1:100 (w/w)). The cleaved products were monitored at several time points. Digestion rates of TNC, MDK, and CYR61 cleaved by KLK7 were compared. The y axis represents peptide abundance, calculated as the ratio of light/heavy peptide area; for A–C the highest ratio observed in each experiment was considered as 1. Peptide abundances were fit to a well established pseudo-fist-order kinetic equation (A/A0 = 1 − e−(kcat/Km×Eo×t) with A/A0 the relative abundance and E0 the enzyme concentration) to determine the activity pattern for each substrate (48). HD = hairpin binding domain. For more details see “Results.”