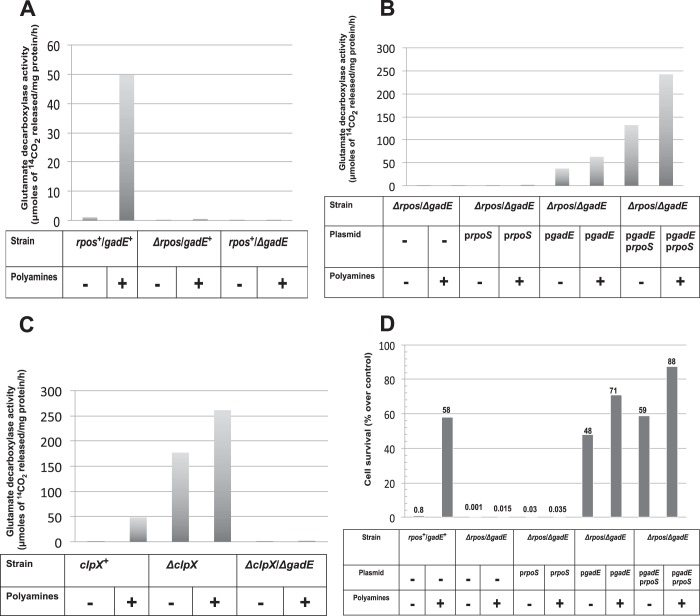
FIGURE 3.
Relative role of rpoS and gadE regulators on polyamine-induced glutamate decarboxylase activities; gadE is the direct regulator of GAD activation and acid survival. A, polyamine mutants (rpoS+/gadE+) and their isogenic rpoS mutant (ΔrpoS/gadE+) and gadE mutant (rpoS+/ΔgadE) cells were grown overnight, and glutamate decarboxylase activities from the cell extracts were assayed as described in the text. B, double mutants of both ΔrpoS and ΔgadE were made in the polyamine mutant background (Table 1), and cells were transformed with either pArpoS, pQEgadE or both plasmids as indicated. Cultures were grown in Vogel Bonner medium (with necessary antibiotics) with or without polyamine supplementation (100 μm spermidine plus 100 μm putrescine) to 0.4 A600. pRpoS plasmid was constitutively expressed, whereas gadE expression was induced by adding 0.5 mm isopropyl β-d-thiogalactopyranoside, and the cultures were grown for an additional 24 h. Cell extracts were made from these cells and assayed for glutamate decarboxylase activity. The results are averages of three different assays expressed in μmol of 14CO2 released per mg of protein per h. C, deletion of either clpX or both clpX and gadE were introduced in the polyamine mutant (HT873). The cultures were deprived of amines and then grown with or without amines overnight for glutamate decarboxylase activity assays as mentioned above. D, the cultures were grown and induced by 0.5 m isopropyl β-d-thiogalactopyranoside as described above in Fig. 3B but with the inclusion of glutamate (300 μm) in the media. At the end of the 24-h incubation, the cells were tested for acid survival at pH 2.5 for 30 min.