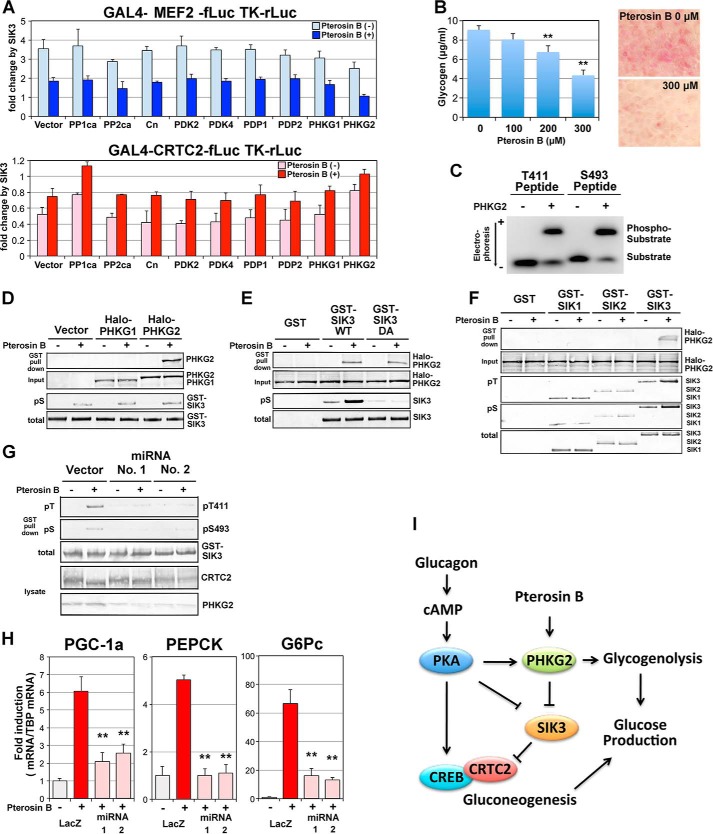
FIGURE 9.
PHKG2 inactivates SIK3 in response to pterosin B. A, a reporter assay was performed in HEK293 cells. Some kinases and phosphatases were overexpressed together with GAL4-MEF2 or GAL4-CRTC2 reporters (see Fig. 2B). The bars indicate fold activation (MEF2) or repression (CRTC2) by SIK3 overexpression (n = 3). In SIK3 without transcriptional regulatory activity, the fold change (activation or repression) approaches 1 (no change). Pterosin B was added at 300 μm. B, AML-12 cells that had been cultured for 72 h with daily medium change were incubated with various concentrations of pterosin B (0–300 μm) for 3 h. The glycogen concentrations were then measured (left panel). Intracellular accumulation of glycogen was observed after periodic acid-Schiff staining (right panel). C, the GST fusion PHKG2 enzyme was overexpressed in COS-7 cells and purified using a glutathione column. Fluoro-peptides corresponding to SIK3 Thr-411 and Ser-493 were incubated with GST-PHKG2 in the presence of ATP. Phosphorylated peptides were separated by electrophoresis on agarose gel. D, GST-SIK3 were overexpressed in AML-12 cells in the presence of Halo-tagged PHKG1/2 and pulldown by glutathione-Sepharose after 3 h of pretreatment with pterosin B (300 μm). PHKG1/2 and phospho-SIK3 (pS) were detected by anti-Halo tag antibody and anti-pS493, respectively. E, GST-SIK3 WT and T411A/S493A mutant (DA) were used. F, GST SIK1–3 were used. G, PHKG2 protein was knocked down in AML-12 cells by transformation with miRNA plasmid vectors. H, the same sequences of miRNA were transferred into an adenovirus vector and knocked down PHKG2 protein in AML-12 cells to monitor pterosin B-induced gluconeogenic gene expression (300 μm, 2 h). n = 3. **, p < 0.01 (compared with the control group, LacZ-pterosin B). I, hypothetical model of SIK3 signaling.