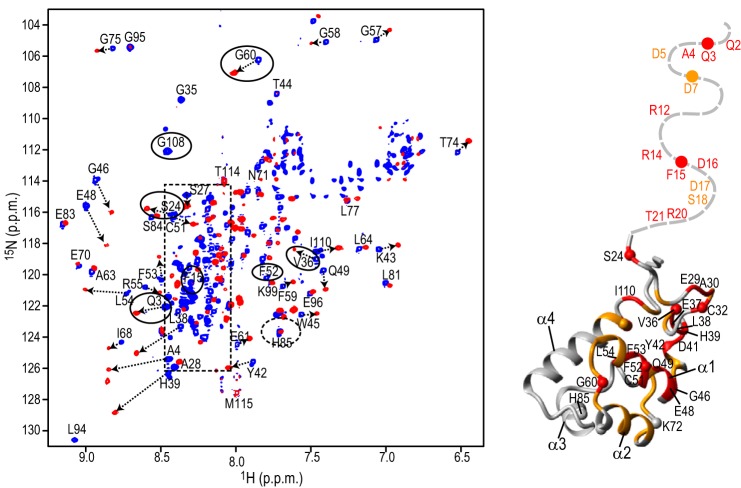
FIGURE 2.
Structural mapping of the DCAF1-VpxSIVmnd interaction onto the SAM domain. A Superposition of the 900-MHz 1H,15N TROSY-HSQC spectra of the MND SAMHD1 NTD (52 μm) in the absence (blue) and presence (red) of 52 μm DCAF1-VpxSIVmnd at 298 K (left panel). Selected assignments are provided for several representative, well separated resonances, labeled on free MND SAMHD1 NTD spectrum. Resonances in DCAF1-VpxSIVmnd-bound state are tentatively assigned and connected to the free resonances by arrows. Resonances of the residues that are different in monkey and human are enclosed by ovals. The random coil region of the spectrum is shown by a dashed rectangle. Structural mapping of residues that exhibit large 1H,15N chemical shift perturbations upon DCAF1-VpxSIVmnd binding is provided (right panel). Residues that experience perturbations >0.15 ppm and between 0.07 and 0.15 ppm are colored in red and orange, respectively, onto the backbone structure of the human NTD region (residues 23–118; PDB code 2E8O). The N-terminal flexible tail (residues Met-1–Pro22) for which no coordinates are available is represented by a gray dashed line.