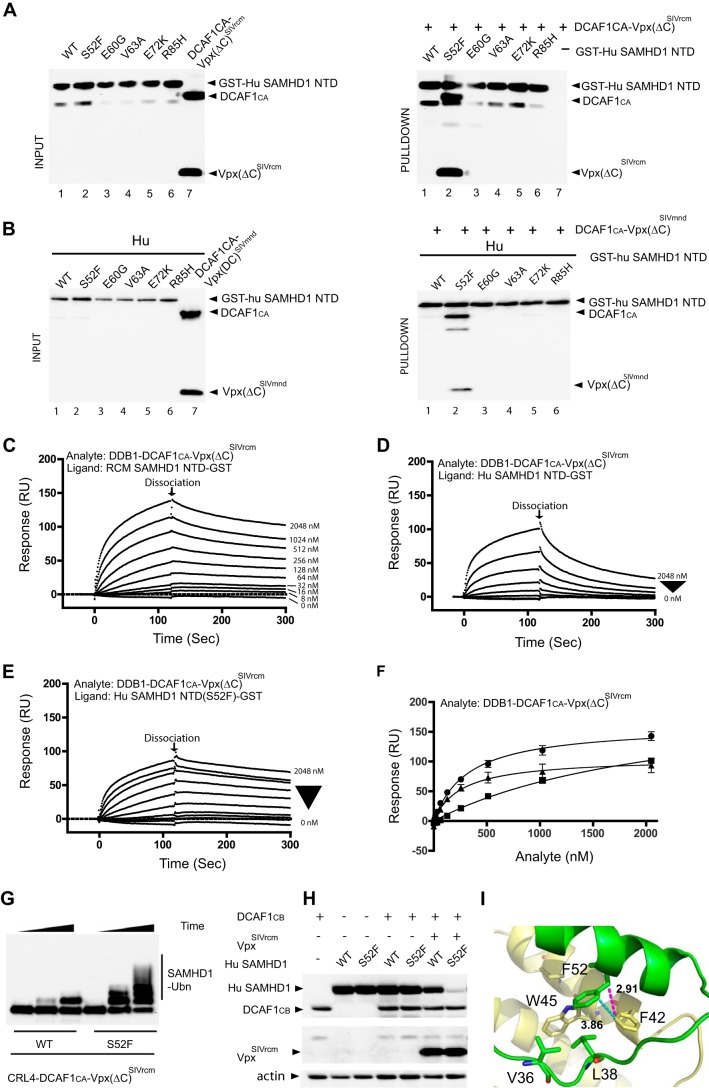
FIGURE 4.
A single residue in the SAM domain of human SAMHD1 is critical for binding to DDB1-DCAF1-VpxSIVrcm and DDB1-DCAF1-VpxSIVmnd. A and B, GST-pulldown assay of GST-Hu SAMHD1 NTD interacting with DDB1-DCAF1CA-Vpx(ΔC)SIVrcm (A) and DDB1-DCAF1CA-Vpx(ΔC)SIVmnd (B). Hu SAM domain residues that differ from the simian protein were individually changed to those present in MND SAMHD1 and RCM SAMHD1 (see Fig. 1A). Pulldown assays of DDB1-DCAF1CA-Vpx(ΔC)SIVrcm or DDB1-DCAF1CA-Vpx(ΔC)SIVmnd protein complexes with GST-Hu SAMHD1 NTD were immunoblotted with the appropriate antibodies. C–E, real-time kinetic analysis DDB1-DCAF1-VpxSIVrcm binding to NTD. SPR sensorgrams of DDB1-DCAF1CA-Vpx(ΔC)SIVrcm binding to RCM SAMHD1 NTD-GST (C), Hu SAMHD1 NTD-GST (D), and Hu SAMHD1 NTD(S52F)-GST (E). The concentrations of DDB1-DCAF1CA-Vpx(ΔC)SIVrcm were 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 nm. F, binding isotherms of DDB1-DCAF1CA-Vpx(ΔC)SIVrcm binding to RCM SAMHD1 NTD-GST (solid circles, Kd = 322 ± 25 nm), to Hu SAMHD1 NTD-GST (solid squares, Kd = 2260 ± 370 nm), and to Hu SAMHD1 NTD(S52F)-GST (solid triangles, Kd = 256 ± 42 nm) were generated by plotting the response levels with different analyte concentrations at 120 s (marked as dissociation in C, D, and E). RU, response units. Dissociation constants were determined from two independent series of experiments. G, in vitro ubiquitination (Ubn) of full-length Hu SAMHD1 WT or S52F with CRL4-DCAF1CA-Vpx(ΔC)SIVrcm E3 ubiquitin ligase. T7-epitope-tagged SAMHD1 proteins were incubated with E1, E2, ubiquitin, and the appropriate E3 ligases in ubiquitination buffer, as described under “Experimental Procedures.” H, HEK293 cells were transiently co-transfected with DCAF1CB (residues 1040–1400), Hu WT or S52F SAMHD1, and VpxSIVrcm as indicated. The levels of expressed proteins were determined by immunoblotting with appropriate antibodies. I, residue Phe-52 of MND SAMHD1 NTD (green, displayed in stick representation) is involved in hydrophobic contacts with Phe-42 and Trp-45 of VpxSIVmnd (yellow, displayed in stick representation).