FIGURE 1.
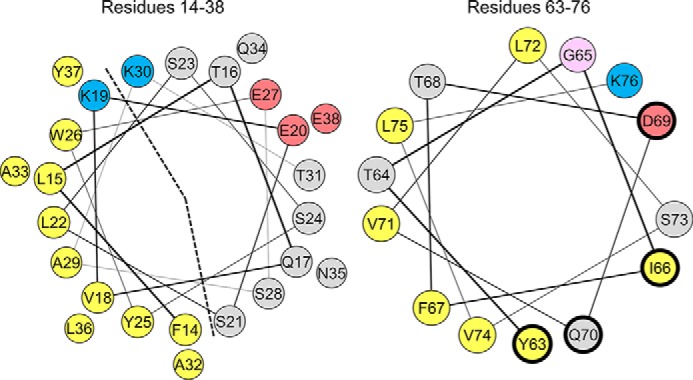
Helix wheel diagrams of the large N-terminal (left) and C-terminal (right) α-helices in lipid-bound apoC-II. The N-terminal helix (left) is a lipid-binding class A amphipathic helix, characterized by an apolar face (marked by a dashed line) of nine hydrophobic residues (yellow), basic residues (blue) at the polar/apolar lipid/water interface, and acidic residues (red) along the polar face. The C-terminal helix (right) is a class G helix, characterized by a random radial distribution of basic (blue) and acidic (red) residues. The four residues that interact with LPL (Tyr-63, Ile-66, Asp-69, and Gln-70) have a thicker, dark blue edge. Polar residues are gray, and glycine is pink.
