FIGURE 1.
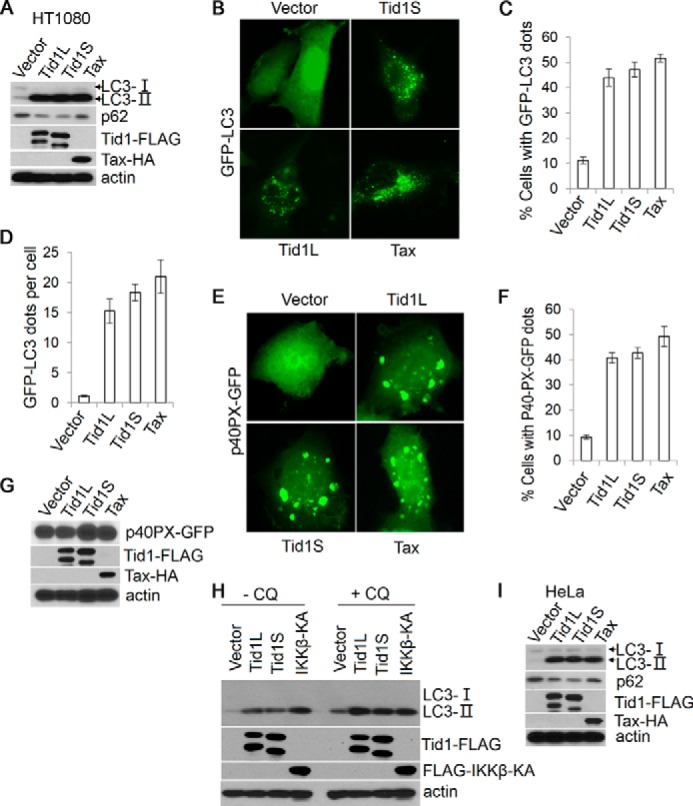
Tid1 induces macroautophagy. A, Tid1L-FLAG, Tid1S-FLAG, or Tax-HA was transiently transfected into HT1080 cells, and total protein lysates were collected 24 h post-transfection and were analyzed with anti-LC3, anti-p62, anti-FLAG (for detecting ectopically expressed Tid1-FLAG), and anti-HA (for detecting Tax-HA). β-Actin was used as protein loading control. B, GFP-LC3 was co-transfected with vector, Tid1L-FLAG, Tid1S-FLAG, or Tax-HA in HT1080 cells. C and D, the formation of cytoplasmic LC3+ foci was examined with fluorescence imaging, and the percentage of cells with LC3+ puncta (C) and average dots per cell (D) were statistically analyzed. p40PX-GFP was co-transfected with vector, Tid1L-FLAG, Tid1S-FLAG, or Tax-HA in HT1080 cells. E–G, the cytoplasmic p40PX-GFP aggregates, the percentage of cells with p40PX-GFP aggregates, and protein expression evaluation with immunoblot are shown in E, F, and G, respectively. H, HT1080 cells were transfected with vector, Tid1L-FLAG, Tid1S-FLAG, or FLAG-IKKβKA. 24 h following transfection, cells were treated with DMSO or with chloroquine (CQ, 50 μm) for 2 h, and total protein lysates were collected for immunoblot analysis with anti-LC3, anti-FLAG, and anti-HA blots. I, Tid1L-FLAG, Tid1S-FLAG, or Tax-HA were transiently transfected into HeLa cells, and total protein lysates were collected 24 h post-transfection for detecting LC3, p62, Tid1-FLAG, and Tax-HA.
