FIG 2.
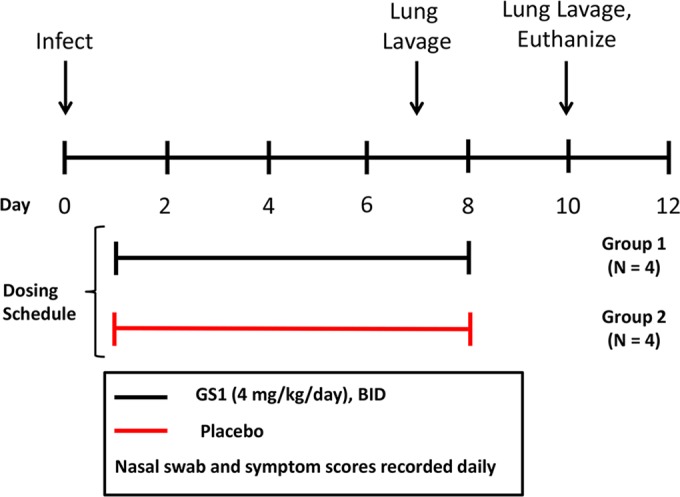
Experimental design for study 1. Calves were randomized into 2 groups based upon weight and bRSV antibody titers. On day 0, animals were inoculated with nebulized bRSV delivered to the respiratory tract through a face mask covering the nose and mouth. At 24 h postinoculation, each group of animals was administered GS1 at 2 mg/kg or placebo by intravenous injection BID for a total of 7 days. Nasal swabs and blood samples were collected daily for 10 days before each dose. On day 7 postinoculation, bronchiolar lavage was conducted on selected animals. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. The animals were euthanized on day 10 of the study, and tissues were collected for necropsy and histopathology.
